Results for Cavendish fruit rejects
Subtropical banana reject analysis
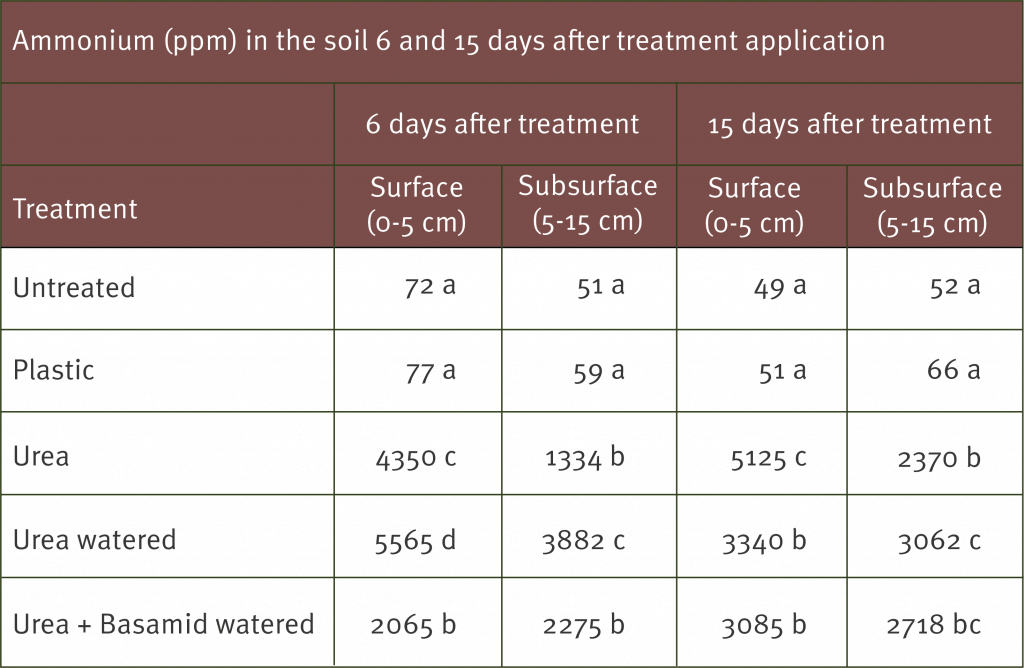
The following are the results for reject Cavendish fruit, assessed as part of the Subtropical Banana Reject Analysis. The figure below shows the proportion of reject Cavendish fruit that fell into each of the three defect categories. As you can see 62% of all reject Cavendish fruit was due to pre-harvest physical defects. This was a far greater percentage than either post-harvest defects (27%) or pest and disease defects (11%). This gives us a good indication where the majority of the damage is occurring and highlights an opportunity for growers to greatly reduce rejects by addressing pre-harvest physical defects.
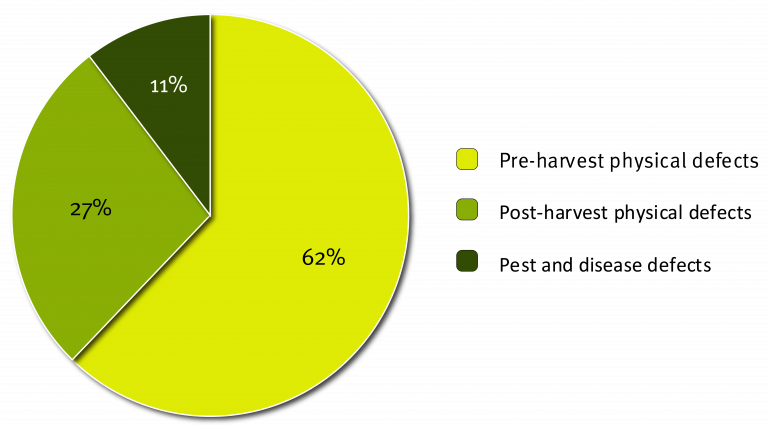
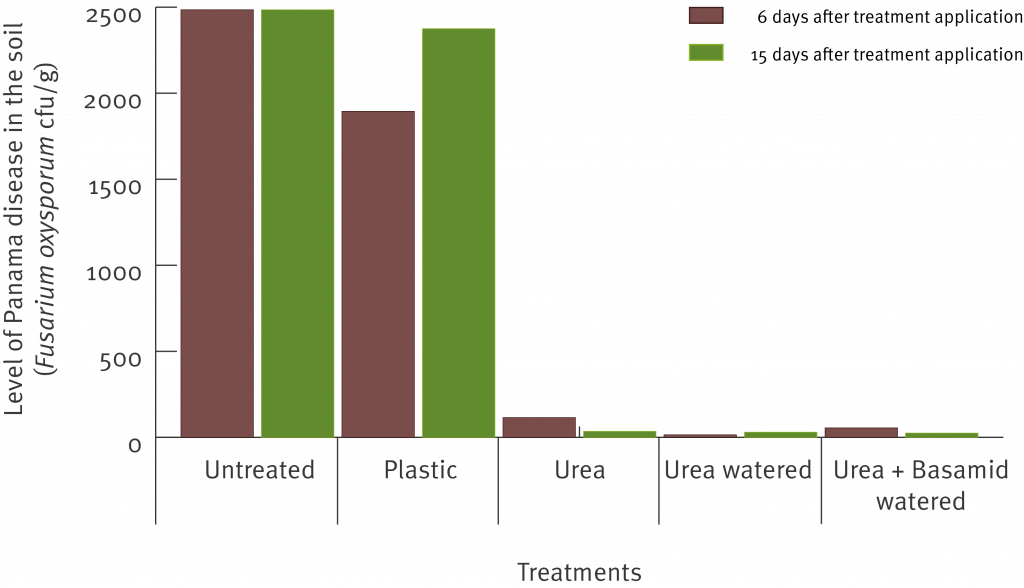
Although this chart provides us with a start, it does not give us the most complete picture. It does not tell us which specific defect types are causing the most rejects or where best to focus efforts to reduce the number of reject fruit. Taking a closer look, the graph below shows the 15 most common defect types in descending order from left to right across all three defect categories, which accounted for 90% of all reject Cavendish fruit in the study.
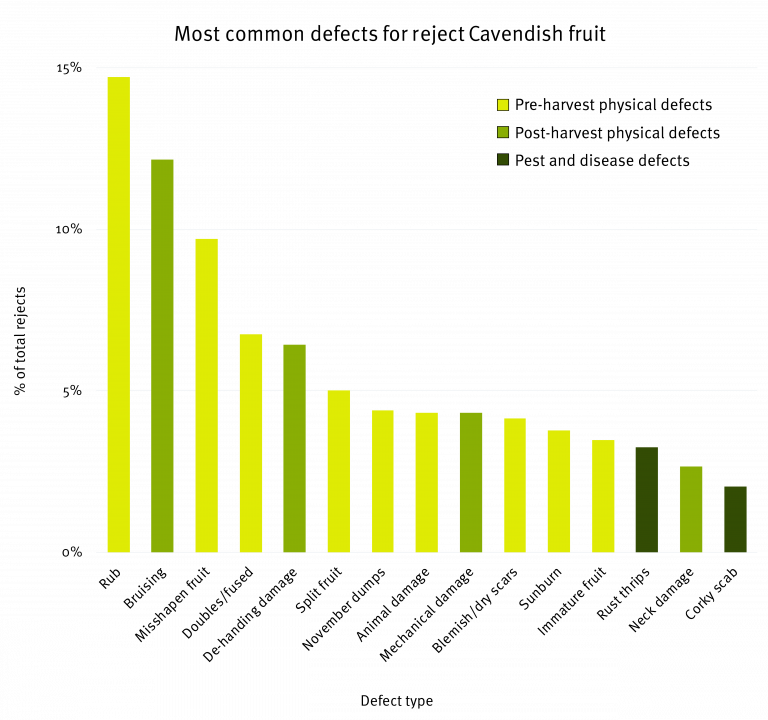


It must be noted that the high proportion of rejects resulting from misshapen fruit is believed to be associated with the dry conditions experienced across the NSW growing regions during the study. Further reject analyses under ‘normal’ growing conditions or over a longer period would need to be undertaken to confirm this result.
Within this list there are some defect types that can be relatively easily improved with changes to pre or post-harvest practices, such as bruising and de-handing damage. Some other defects such as misshapen fruit, fused fruit and November dumps are caused or attributed to factors that we have limited control over (e.g. environmental conditions). This list provides us with the information we need to be able to prioritise the development of research, development and extension activities aimed at reducing Cavendish rejects in the subtropics.
A poster is now available showing common quality issues and packing guidelines for subtropical banana growers. To receive a hard copy or for more information contact NSW DPI Industry Development Officer Tom Flanagan on (02) 6626 1352 or email tom.flanagan@dpi.nsw.gov.au

NSW DPI would like to acknowledge all growers who agreed to participate in the study, Matt Weinert, Leanne Davis from NSW DPI and Valerie Shrubb from AGRIC for undertaking the research.
This research has been funded as part of the Subtropical Banana Development and Extension Program (BA16007), which is funded by Hort Innovation, using the banana research and development levy and co-investment from the New South Wales Department of Primary Industries and WA Department of Primary Industries and Regional Development. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.