Chemical treatment of spider mites
Restricting the use of chemicals that cause mite population flares
Some chemicals are associated with mite flares. This can be due to several reasons but primarily it is because these chemicals either encourage the mites to lay more eggs (the neonicotinoids, e.g. imidacloprid) or eliminate natural predators (the synthetic pyrethroids, e.g. bifenthrin). Where possible, avoid using these chemicals or if they must be used, time their use to the low-risk periods for mite flares, such as winter.
Correct application of miticides
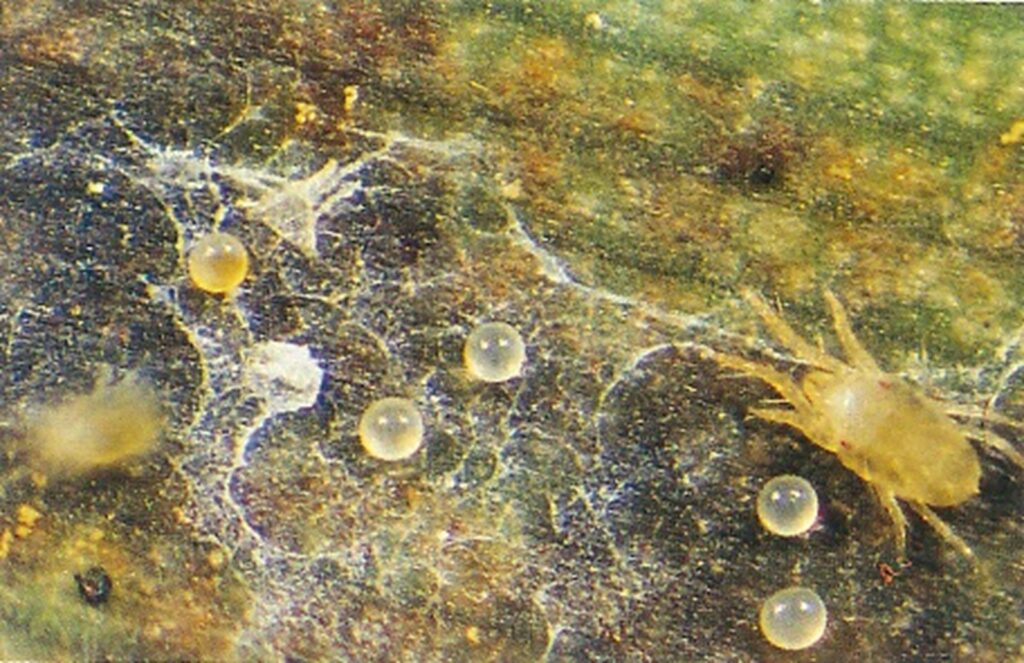
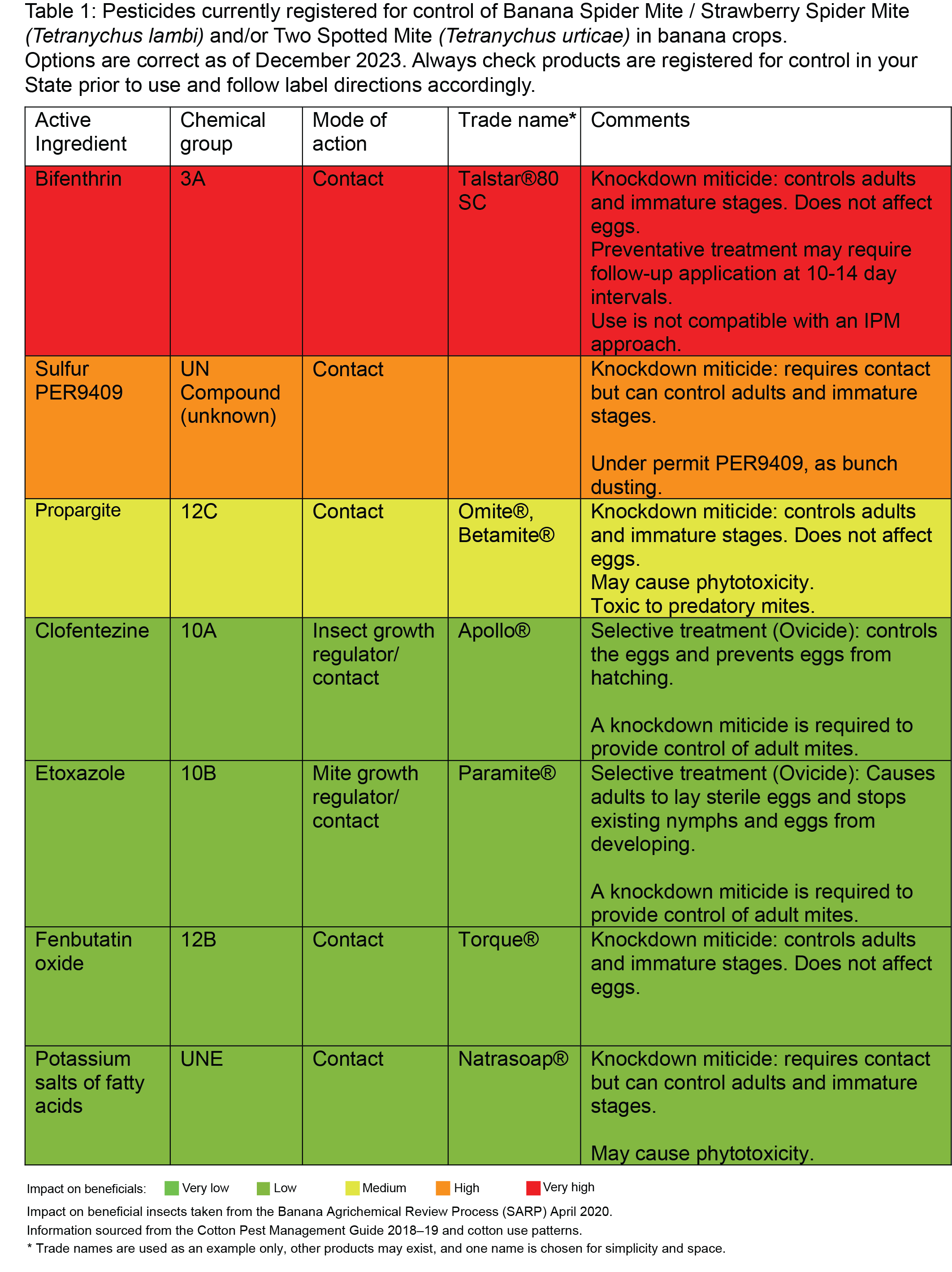
Firstly, it’s important to check to ensure that live mites are still present and it’s not residual damage that’s still visible. With only a limited number of miticides available to the banana industry, it is important for treatment efficacy and long-term availability of these products that they are applied correctly.
- Avoid using neonicotinoids for control of Banana weevil borer and Banana rust thrips (e.g. imidacloprid), particularly if hot dry conditions are expected. These chemicals can cause mite flares as they encourage mites to lay more eggs.
- Avoid using broad spectrum pyrethroids (e.g. bifenthrin) as these products will remove the predator (beneficial) population. There is also the risk of spider mite populations developing resistance to these chemicals.
- Miticides will not provide instant results and monitoring after spray applications is required as it may take 2-3 days before the mites begin to die.
- Apply miticides in the cooler parts of the day as the leaves will close up during the middle of the day making full leaf coverage difficult to achieve. Mites are generally found on the undersides of the leaves therefore it is important that the leaves are open at the time of application.
- Apply miticides with at least 400 L/ha and up to 600 L/ha of water to ensure good coverage. Poor coverage will result in limited mite mortality and may create chemical resistance problems.
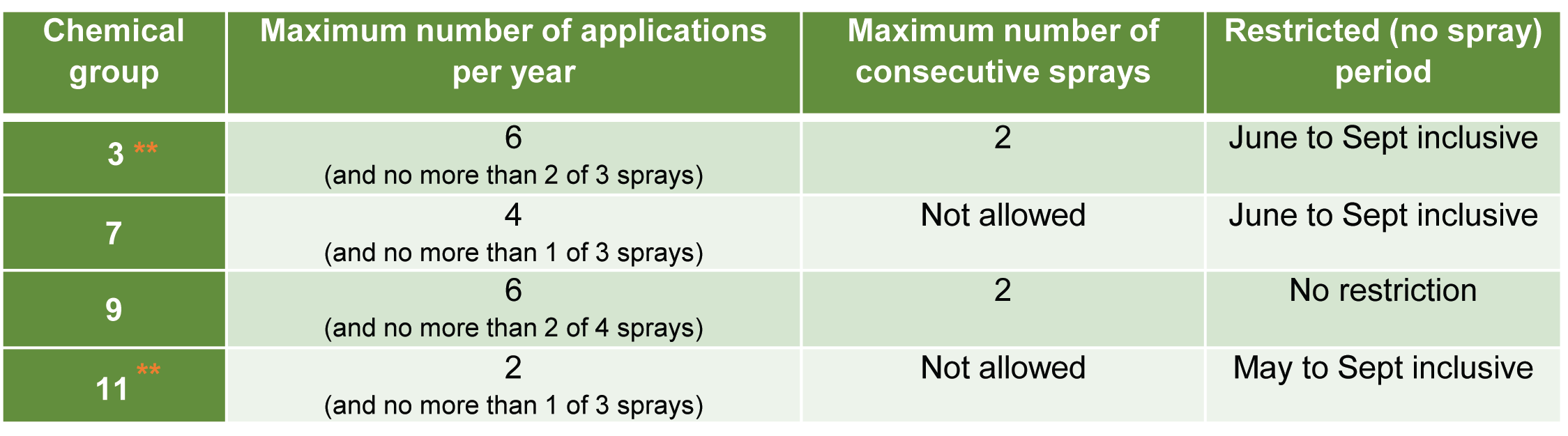
- Rotate between the available modes of action, chemical groups and abide by the restricted number of annual uses for each product to minimise the chance of chemical resistance issues.
- Knockdown miticides (e.g. fenbutatin oxide) will only control nymphs and adults and therefore may require a follow up application 10–14 days later to control mites that have hatched from the eggs.
- Some miticides are referred to as ovicides (e.g. clofentezine) meaning they only control the eggs, preventing them from hatching. These must be applied with a knockdown miticide to control the adult population. Follow label instructions for resistance management as ovicidal miticides have a history of resistance development if over used.
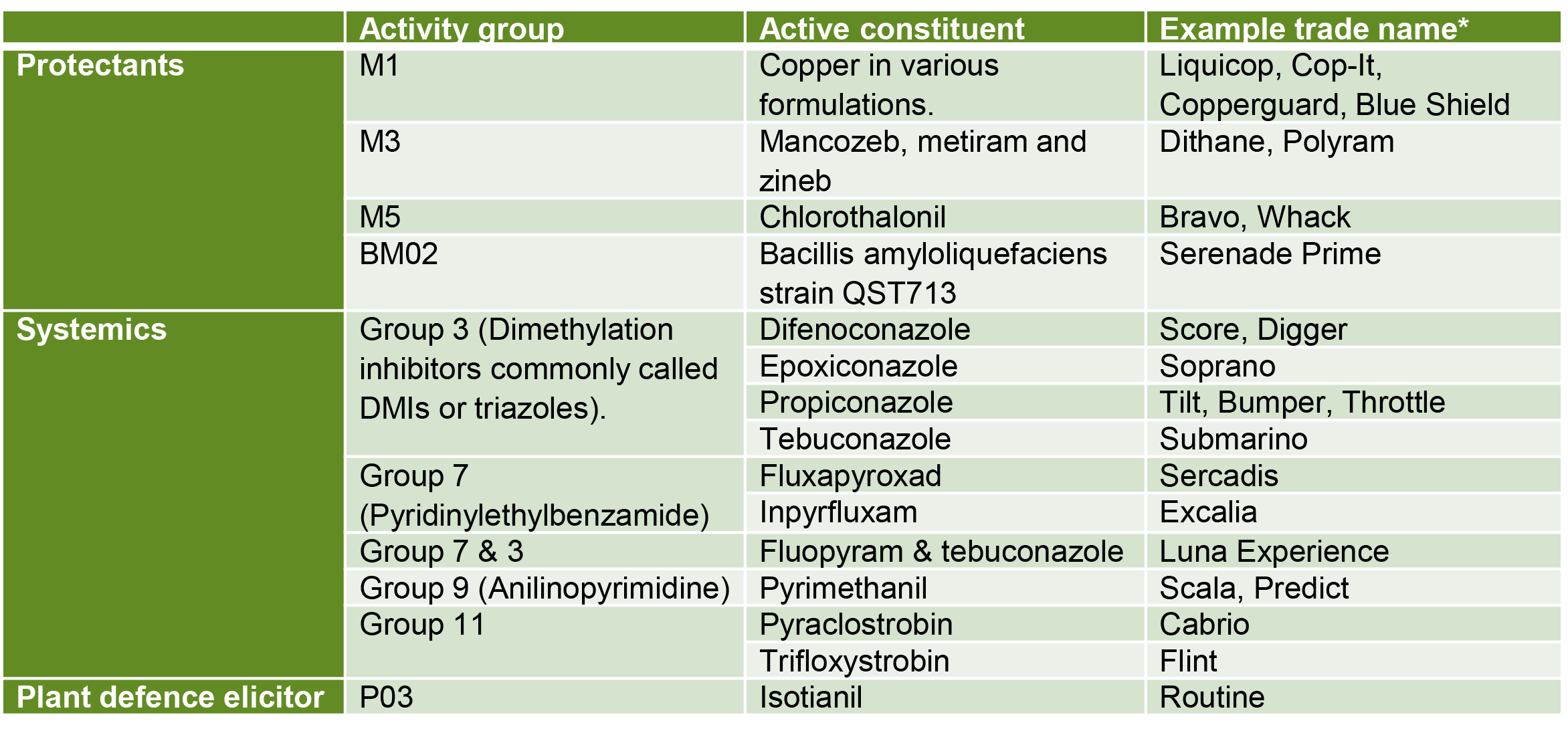
Actives registered for control of spider mites in bananas
Always check the current registration status of chemicals before use by visiting the Australian Pesticides and Veterinary Medicines Authority website (Click here) and always follow label directions.
For more information contact
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland and Treverrow, N., Pearley D., and Ireland, G 1992 Banana weevil borer : a pest management handbook for banana growers. : NSW Agriculture, North Coast Region; NSW Banana Industry Committee; Horticultural Research & Development Corporation.
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.