More varieties showing resistance to TR4 in NT trials
By Sharl Mintoff, Samantha Bond, Chris Kelly, Maxine Piggott, NT Department of Industry, Tourism and Trade, Darwin; and Jeff Daniells, Queensland department of Agriculture and Fisheries, South Johnstone.
Seven varieties in a banana variety trial in the Northern Territory have demonstrated TR4 resistance, in the plant crop, as good or better than that of Goldfinger.
In December 2021 we described a new TR4 varietal screening trial (see here), which had commenced in December 2020 and ran for the final 12 months of the project- Improved plant protection for the banana industry (BA16001). Over the 12 months plant crop data was collected to determine the TR4 reaction of several new varieties that had become available for evaluation from plant breeding programs.
Overview
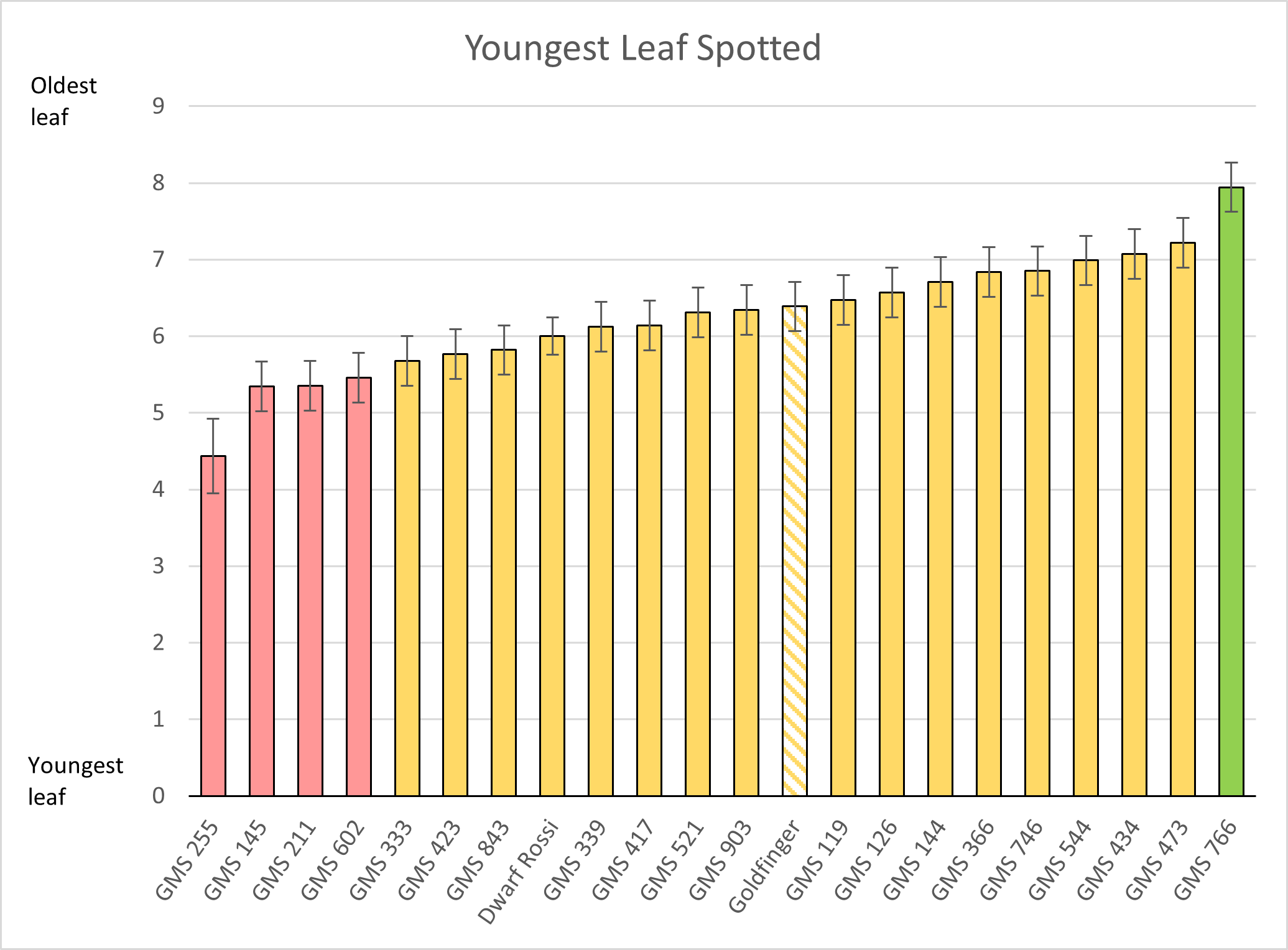
In this trial, 24 varieties were screened for resistance to TR4, and included three Cavendish selections, four novel hybrids from the CIRAD program in the French West Indies, four Lady Finger hybrids from the EMBRAPA program in Brazil, some parental lines used in the breeding program and three Goldfinger mutant selections.
The Goldfinger mutant selections were generated as part of an earlier project (BA14014) in Queensland, to improve the eating characteristics of Goldfinger, whilst hoping to retain its TR4 resistance in the process (see more here).
Methods
As per the previous trials, all plants were artificially inoculated at planting with millet colonised with TR4. Disease assessments commenced at the first signs of external disease symptoms, with assessments occurring every two weeks, taking note of the presence of external and internal symptoms. The trial included three reference varieties of known susceptibility or resistance to TR4;

- Williams - Very Susceptible
- Formosana (GCTCV 218) - Intermediate
- Goldfinger (FHIA-01) - Resistant
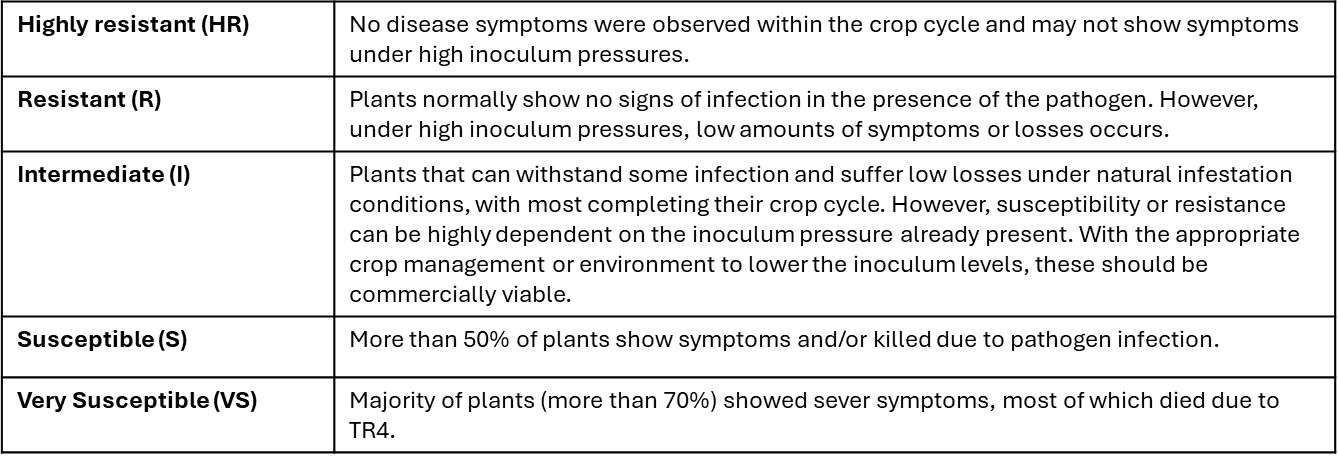
The disease resistance of each variety was determined by scoring the severity of the disease and by grouping them into one of the following categories:
Results
Highly resistant and resistant
Parental lines M61 and Calcutta 124 both rated as highly resistant, as did the True-to-type Asia Pacific #1 and CIRAD hybrid X17. Encouragingly, two Goldfinger mutants 144 and 417, both showed no signs of infection by TR4. The Goldfinger reference material and Goldfinger mutant 544 both fell into the resistant category as a low amount of disease was noted in a small number of plants.
Intermediate
Cavendish varieties Formosana (Intermediate reference control) and Short Fruit Williams (Williams off-type) both fell into the intermediate rating. As did the EMBRAPA Lady Finger hybrid PA12.03, the Highgate hybrid 2390-2 and Yangambi km5.



Susceptible and very susceptible
Cirad hybrid lines 925, 918 and L9 all displayed susceptibility to TR4 in the plant crop, as did the EMBRAPA lines PV03.44, JV42.41, PA03.22 and the Highgate hybrid Buccaneer. Two Cavendish varieties Williams and GCTCV 106 selection both rated as very susceptible to TR4.
Although this trial only ran for the plant crop cycle, some interesting results were obtained. The TR4 resistance of the Goldfinger mutants is encouraging, as they were originally selected for their improved eating characteristics and appear to have retained their resistance to TR4 as hoped. The true-to-type Asia Pacific #1 had not yet completed harvest in the plant crop at the time the trial had to be wound up. However, it had no symptoms of TR4 externally or internally.
Interestingly, the Short Fruit Williams, showed a similar intermediate disease reaction to that of Formosana. Short Fruit Williams is, as the name suggests, a selection of Williams which has shorter fruit. It had occurred as a tissue culture off-type in north Queensland. We included it in this screening because it had some traits in common with TR4 resistant selections from Taiwan, noticeably its slightly longer crop cycle. This could indicate an association of certain characteristics, such as selections with slower crop cycles, possessing TR4 resistance, and thus the ability to locate potentially resistant variants when TR4 is not present.
Plant crop disease ratings of assessed varieties
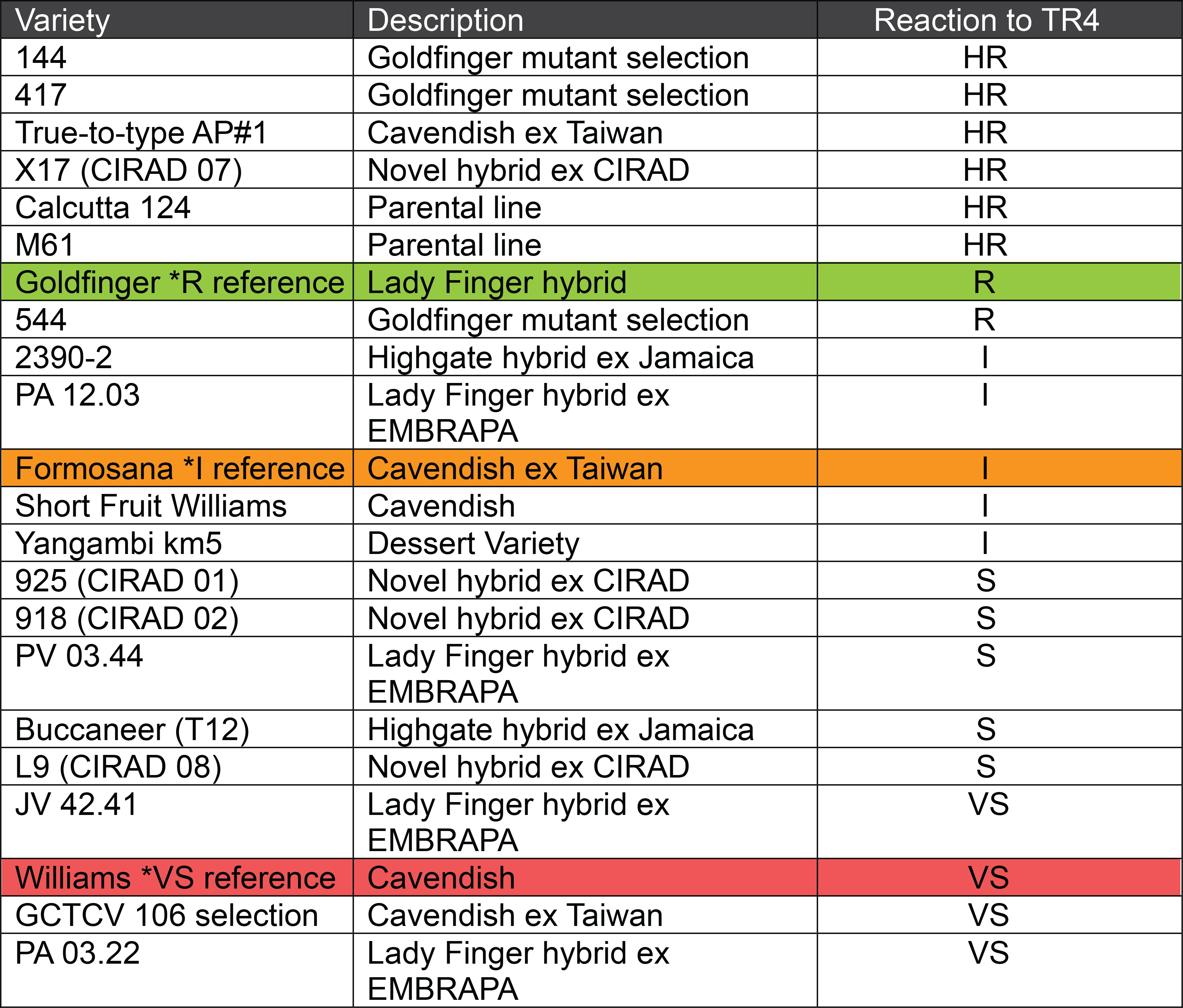
HR = Highly Resistant, R = Resistant, I = Intermediate, S= Susceptible, VS= Very Susceptible
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and the Northern Territory Department of Industry, Tourism and Trade and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.