Agronomic evaluation of new varieties South Johnstone screening trials (2022)
By Jeff Daniells and Katie Robertson
About the trial
Five new TR4 resistant Cavendish selections from Taiwan and eighteen TR4 resistant Cavendish from the Department of Agriculture and Fisheries’ mutagenesis program, were field planted at South Johnstone and are being assessed for agronomic performance. Some new Lady Finger types from Brazil are also included in a smaller subtrial.

Objectives
The Department of Agriculture and Fisheries imported new varieties as part of the ‘Improved plant protection for the banana industry’ project (BA16001) completed in 2021. These varieties included some TR4 resistant Cavendish from Taiwan and various Lady Finger types from Brazil, which were released from quarantine in early 2022. Also during that project, 18 TR4 resistant Cavendish selections were made from the mutagenesis trials established earlier in the Northern Territory as part of the ‘Fusarium wilt Tropical Race 4 research program’ (BA14014).
The new trial at South Johnstone was field planted with these varieties in early December 2022. They will be evaluated for agronomic performance over two crop cycles as part of the project ‘New varieties for Australian banana growers’ (BA21002). This is a first look at many of these varieties to see how they perform under north Queensland conditions. In addition, preliminary taste panel assessments will be made.
In conjunction with this agronomic evaluation, several of these varieties are to be screened against TR4 in the Northern Territory to confirm their level of disease resistance. However, the banana freckle outbreak in the NT last year is contributing to delays in commencing this component of the broader work. The Lady Finger types will also be evaluated for Race 1 Panama disease resistance in an on-farm trial on the Atherton Tablelands.
Overview of varieties
There are five new Cavendish selections from Taiwan including Improved Formosana, which is reported as quicker cycling than standard Formosana and shorter in stature, and GCTCV 219 which has sweeter fruit.
There are eighteen Cavendish selections from DAF’s mutagenesis program. They were derived from the already TR4 resistant CJ19 and GCTCV 215. The selections were made for improved agronomic characteristics, including plant stature, and having a cycle duration closer to Williams.
Ones to watch amongst the Lady Finger types from Brazil are SCS451, a selection of Santa Catarina Prata with tolerance to Race 1 Fusarium wilt, and the Sugar hybrid, Princesa.
Observations and results
Observations and results are now available for:

More information will be made available as the trial progresses.
This research has been funded as part of the project New varieties for Australian banana growers (BA21002), which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Seasonal forecasting
Finger on the pulse with the latest climate and seasonal forecasting information
By Shanara Veivers
Weather data and information is an absolute necessity for any farmer to consider in making critical on-farm decisions. Those decisions can include when to plant, spray, irrigate and fertilise your crop. Even successful transportation of your crop can be dependent upon the cooperation of the weather. Having the advantage of knowing climate and seasonal conditions for your location in advance can be a significant benefit in protecting your crop and property.

Organised as part of the National Banana Development and Extension Project, a climate and weather workshop was held for the NextGen banana growers’ group and interested industry stakeholders at the Department of Agriculture and Fisheries (DAF) South Johnstone Research Station.
If you missed it, the workshop kicked off with participants reflecting on notable weather events and natural disasters experienced in the Cassowary Coast region from the early 1990’s to present.
A series of interactive presentations, incorporating videos about important climate drivers and the new Bureau of Meteorology (BOM) forecasting tools and outlooks were presented by Dr Neil Cliffe (Manager for the DAF Drought and Climate Adaptation Program). Dr Cliffe spoke about important climate drivers that influence Australia’s highly variable climate.
Check out the Bureau of Meteorology video links below which explain how these drivers influence Australia’s climate:
1. El Nino-Southern Oscillation (ENSO) – One of Australia’s strongest climate drivers.
2. Madden Julian Oscillation (MJO) – An important driver of tropical weather around the globe.
3. Indian Ocean Dipole (IOD) – Most significant climate driver in the Indian Ocean. It refers to the year-to-year changes in tropical sea temperatures in the Western and Indian Oceans.
Tools
The BOM have developed new forecasting tools to assist farmers and agribusiness plan for extreme weather events and build stronger climate resilience into farming systems. These tools provide forecasts for 2 weeks to 3-month timeframes, filling a forecast gap not previously covered in the existing shorter and longer-term forecast products. Five new features on the BOM website include:
1.
Maps showing the likelihood of having extreme rainfall, maximum and minimum temperature for the weeks, months, and seasons ahead.
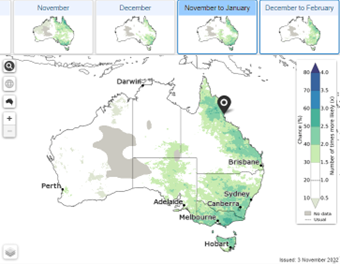
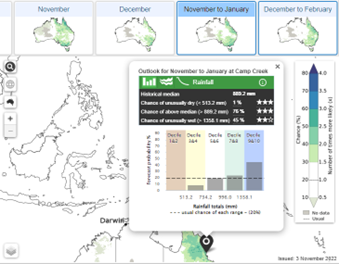
2.
Location specific data indicating shifts in rainfall and temperature probabilities compared to usual across the deciles/quintiles.
3.
Climate summary – Forecast timeline showing what has happened over the last few months, and what may happen in the next few months for rainfall and temperature.
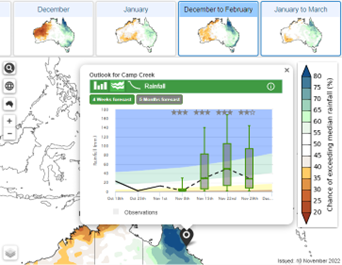
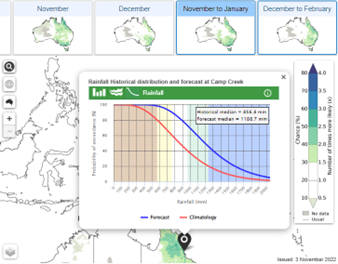
4.
Probability of exceedance (rainfall only) shows the forecast chance of any rainfall total for any location.
5.
Three-day-burst forecast shows the chance over a 3-day period of getting a burst (15, 25, 50 or 75mm) of rainfall in total over those 3-days.
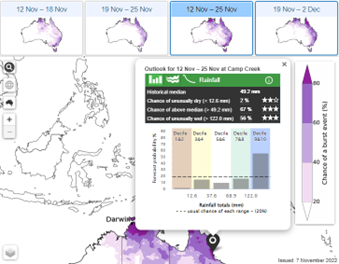
Summary of extreme temperature, water and wind impacts on bananas
High temps
34oC or higher – leaf scorching, root growth stops & under peel discolouration occurs.
Other high temperature impacts – sunburn to stalk, fruit, and cigar leaf (most susceptible), impacts on bunch formation and differentiation.
Low temps
14oC or less – field chilling causing under-peel chilling & root growth stops.
Other low temperature impacts – water-soaked necks on fingers, frost damage, ‘winter yellows’ on leaves, and ‘November dumps’ on bunches.

Waterlogging
More than 24 hours kills root tips; more than 4 hours reduced nutrient loading to 36%.
Other impacts – encourages production of aerenchyma tissue production in roots which improves gas exchange and potentially easier for Fusarium wilt fungi to infect roots.
Water deficit
Controlled deficits experiments have shown reduced productivity by 30-50%.
Other impacts – plant stress; increased pest mites e.g., banana spider mite.
Wind
Highly susceptible to wind damage and don’t need cyclonic winds to produce damage.
Greater than 50km/h can blow down large, bunched plants.
Greater than 70km/h can cause 50-100% blow down.
Participant feedback
Growers and stakeholders who attended the workshop spoke highly of the event and took away knowledge that they felt will help them with making farm management decisions.
“It was one the workshops that I’ve attended and enjoyed most. There was really good information, and I would attend an event like this again”.
“It is hard to get away from the farm sometimes, we are all busy, but we need to make effort to go to these things”.
“Some of the new tools can be used as a pre-emptive strategy, mapping out weather predictions and forecasts and its potential impact on fruit quality”.
“Great workshop and I really enjoyed the activity where we went through the historical data and made a timeline of weather events. Looking back at the historical data set the scene and was good information”.
We will continue to seek input from the NextGen group and other growers to drive the agenda for workshops, meetings and/or activities, to ensure they are relevant and useful. Topics and ideas suggested by participants at this workshop included: business management, leadership, banana agronomy and chemical impacts on bananas. These will be considered for future NextGen and wider industry activities.
The weather workshop was delivered as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Banana flower thrips
Flower thrips
Banana flower thrips (Thrips hawaiiensis) are a tiny pest frequently present in banana bunches. For most growers in Far North Queensland flower thrips are not the main bunch pest which leads to economic losses. However, large enough infestations can cause damage to fruit that does not meet market specifications. Damage caused by flower thrips is more significant in warm dry conditions with lower relative humidity, such as South East Queensland and northern New South Wales.

Correct and timely bell injection is critical for the control of flower thrips. It must be performed when the bell is upright to ensure the insecticide solution provides protection to the entire bunch. Flower thrips are from the same family as Banana rust thrips (Chaetanaphothrips signipennis). Unlike banana rust thrips, flower thrips spend their entire life cycle on the banana plant, therefore, soil treatment does not provide control of flower thrips.
Flower thrips cause damage to the peel of banana fruit from feeding and ovipositing (egg laying). These ovipositions resemble minute raised pimples on the young immature skin. These are readily seen because of a dark raised centre and can be confirmed by lightly touching the raised area with your fingertip. These oviposition marks almost disappear as the fruit matures. However, extensive damage from feeding from adult flower thrips can cause superficial scarring known as ‘corky scab’. This damage is usually confined to the lower hands (as flower thrips damage increases on lower hands as populations increase as they move down the bunch if it hasn’t been treated). Usually it is first noticeable on the outer whirl, where the neck meets the cushion, but can extend to the outer curve of the fruit.


More information
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Banana flower thrips – general information and management
Banana flower thrips Thrips hawaiiensis
General information and management
Occurrence and seasonality
Banana flower thrips are common in banana flowers and among the fingers of newly emerged hands. This pest is found anywhere bananas are grown but its damage is more significant in the less humid, warm and dry climate of South East Queensland and northern New South Wales. Flower thrips are active throughout the year, with increased activity in January through to April. However, as long as flowers are present they can continuously breed.

Description and life cycle
Female flower thrips cause the most damage. They are 1mm long with a pale brown head and thorax and have a black abdomen. They are generally found sheltering under the bracts or inside the flowers. Male flower thrips are smaller (about 0.7mm long), uniformly cream coloured and tend to occur on the outer surface of the bracts.
Adults and nymphs are found on newly emerged bunches and invade the fruit early when the bunch is still covered by its bracts. Recent work has found that flower thrips are present very early in bunch development, inside the bell whilst it is still upright. Flower thrips breed all year round if flowers are present and migrate progressively down the bunch as bracts lift. The lifecycle takes about three weeks in summer, with full development from the egg to the adult taking place on the bunch or in other parts of the plant.
Damage
Damage to fruit is the result of superficial scarring caused by feeding and ovipositing (egg laying). Oviposition damage resembles minute raised pimples on the young immature fruit skin. These have a dark raised centre and can be confirmed by lightly touching the raised area with the fingertips.
Extensive feeding damage causes a ‘corky scab’, a slightly raised grey corky skin covering. This damage is usually confined to the lower hands (flower thrips damage increases on lower hands as populations increase as they move down the bunch, if it is untreated). Usually, it is first noticeable on the outer whirl, where the neck meets the cushion, but can extend to the outer curve of the fruit.


Monitoring
If flower thrips damage is being picked up in the packing shed, the best solution would be to speak to your bell injectors, revise training and ensure that bell injections are being performed correctly (right time and right position).
Flower thrips damage is easy to find in the shed, but it is also possible to find it out in the field. Monitoring for early detection of flower thrips can be done by examining bells of bunched plants every time you’re in the paddock. When large numbers of flower thrips are present, they cause bract-feeding patterns which are ‘lace-like’ in appearance and are lighter than the mauve bracts. These are more pronounced at trimming (when flower thrips populations are highest) and although bunches may already have damage, it gives you an early indication of high pest pressure allowing you to revise training with bell injectors, approximately 12 weeks earlier if assessing damage at harvest in the shed.

Control
Chemical
Chemical control is best achieved with correct bell injection. Flower thrips can make their way between the bracts into the bell and damage very young fruit before bell injection. Therefore, to limit the extent of this damage, the timeliness of bell injection is important. Inject bells whilst still upright. Increase the frequency of bell injecting during warmer months to account for increased plant growth (if possible, as short as every 4-5 days and extending out to every 7 days in winter). Always check the APVMA website for current chemical registrations before use. Below are insecticides currently registered (March 2023) and permitted for bell injection to control flower thrips.
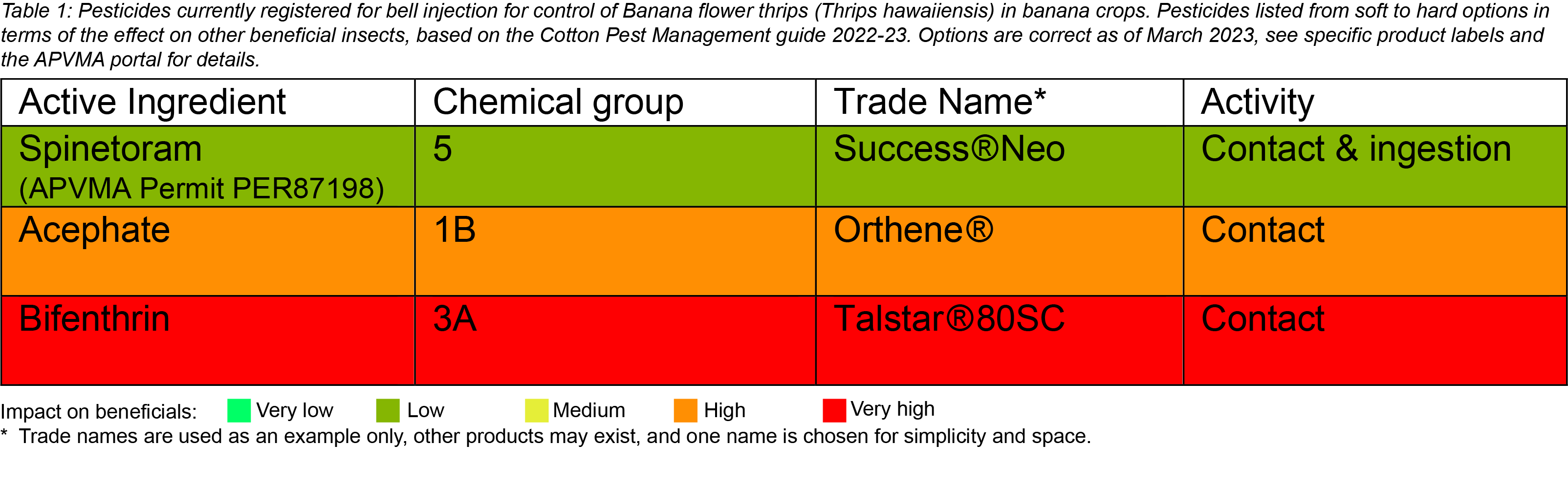
Biological
A range of predatory bugs, predatory mites, ladybird beetles and lacewings can assist in reducing the build-up of flower thrips. Choosing chemical products that are less likely to kill these beneficial insects may assist in suppressing background pest populations.
More information
The Better Bananas team Department of Agriculture and Fisheries South Johnstone 07 4220 4177 or email betterbananas@daf.qld.gov.au
This information is adapted from; Pinese,B., Piper, R 1994, Bananas insect and mite management, Department of Primary Industries Queensland
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Agronomic evaluation of new varieties – South Johnstone screening trial (2020)
Latest update...
Evaluation of the 2020 variety screening trial is now complete. Assessment of the plant and first ratoon crops is now complete and results are now available (see here). Trends which were observed in the plant crop (see here) continued in the first ratoon. Williams outproduced the TR4 resistant Cavendish varieties. All the Lady Finger selections performed well compared to the standard Lady Finger, with either improved plant or bunch characteristics and no yield reduction.
About the trial
This agronomic evaluation screening trial was planted at South Johnstone Research Facility in October 2020. Building on previous variety evaluation work, the trial is looking at new introductions that may have commercial potential for the Australian banana industry, forming part of the recently completed project ‘Improved plant protection for the banana industry (BA16001)’.
Assessment of agronomic traits is being collected over a plant crop and first ratoon with leaf spot screening performed in a nurse-suckered third crop cycle.


Trial five months after planting in March 2021.
Below is a video of a field walk of the evaluation trial taken in September 2021.
About the varieties being evaluated
Three Cavendish selections, four CIRAD hybrids, and six Lady Finger selections were included in the evaluation trial, including two varieties with TR4 resistance.
Observations and results
Observations and results are now available.
What's next?
Currently, screening is underway to evaluate these selections for their tolerance to yellow Sigatoka. Plants will be assessed over three months during the wet season (March, April, and May 2023) and compared against control varieties with known resistance/susceptibility to this fungal leaf disease.
In December 2022, a new variety evaluation was planted as part of the project ‘New varieties for Australian banana growers (BA21002)’. This will again look at the agronomic traits as well as disease tolerance of recently imported varieties.
More information...
If you would like further information, feel free to contact the Better Bananas team via email at betterbananas@daf.qld.gov.au.
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Agronomic evaluation of new varieties South Johnstone (planted October 2020) Plant crop observations and results
Plant crop results - agronomic evaluation trial (October 2020)
Two TR4 resistant selections were among the new varieties being assessed for agronomic performance at the South Johnstone Research Facility. Those who attended the September 2021 field walk saw the varieties as they were approaching harvest in the plant crop (available to watch online here). The results of the plant crop are presented below.
By Katie Robertson, Jeff Daniells and Ashley Balsom
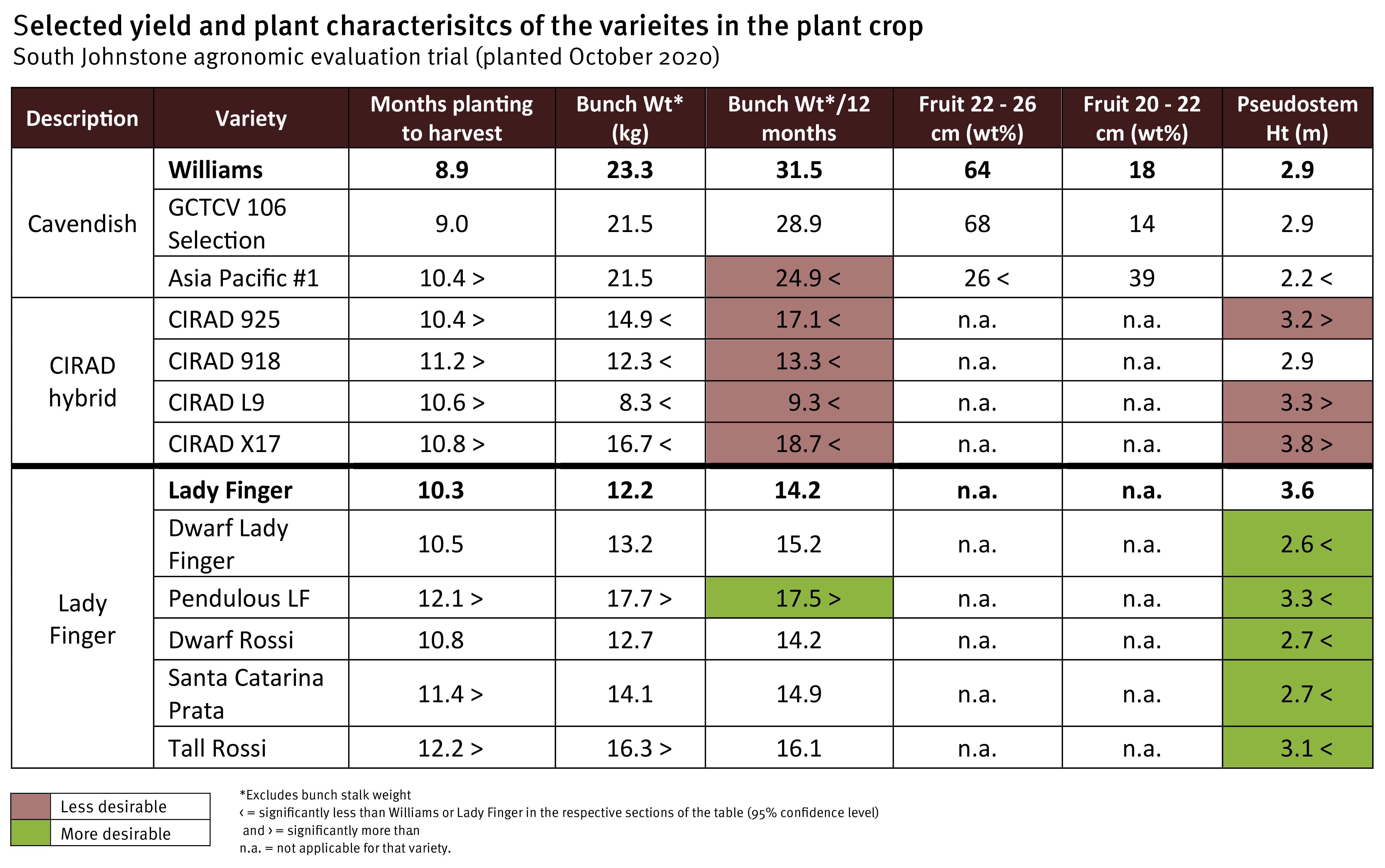
The agronomic characteristics of the new varieties are being compared with industry standards over a plant crop and first ratoon. Three Cavendish selections, four CIRAD hybrids, and six Lady Finger selections were included in the evaluation. The trial was established in October 2020, and bunch harvest was spread from July to November 2021 for the different varieties.
Within the Cavendish varieties, Williams and the GCTCV 106 Selection had the fastest cycle times (planting to bunch harvest) of 8.9 and 9.0 months, resulting in yields of 31.5 and 28.9 kg/year, respectively. In contrast, Asia Pacific #1, a TR4 resistant selection from Taiwan, took 1.5 months longer to reach bunch harvest than Williams, resulting in a 21% yield reduction (24.9 kg/year). This longer cycle time has been a common feature of other TR4 resistant Cavendish varieties previously evaluated. Asia Pacific #1 was 24% shorter in stature than the other Cavendish varieties, standing at 2.2 metres. Fruit length was also shorter. Only 26% of its fruit was in the 22–26 cm finger size category compared to 64% for Williams.
The dwarf selections of Lady Finger (Dwarf Rossi, Dwarf Lady Finger, and Santa Catarina Prata) were 25–28% shorter in stature than standard Lady Finger at bunching. Pendulous Lady Finger (PLF) and Tall Rossi were also shorter than regular Lady Finger by 7 and 13%, respectively. Additionally, PLF had an 18% yield increase per year and displayed much better bunch conformation than Lady Finger’s typical sub-horizontal bunch angle.



The French CIRAD hybrids were developed to have resistance to Sigatoka leaf diseases. One of them, CIRAD X17, has also demonstrated to be highly resistant to TR4 in the Northern Territory screening trials (Read here, pages 18 -19). Like other CIRAD hybrids we have evaluated previously (Read here, pages 16 – 17), these four selections had long, narrow leaf petioles which are brittle and prone to breaking. Although the height of CIRAD 918 was comparable to Williams (average height of 2.9 m), its pseudostem snapped before harvest for 40% of the plants. There were no notable losses from snapping or rolling out in the other three hybrids. These varieties were bred and selected in a tropical region, and they do not seem to handle the relatively cooler winters and some other environmental stressors of north Queensland particularly well. There were also inconsistencies with the rate and capacity of fruit filling. The CIRAD hybrids took 14 – 21% longer to reach harvest maturity and had bunches that yielded 41 – 70% less than Williams per year.

More information
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Maturity bronzing
Maturity bronzing - stretching the limits on fruit quality
By Ingrid Jenkins and Jeff Daniells
Several growers have reported a higher incidence of fruit affected by maturity bronzing in early 2023. In response to grower enquiries regarding what causes maturity bronzing and how to reduce its impact, it seems timely to give an overview of past research and recap on the best management options available to growers.
Maturity bronzing has been a long-term problem for Australian commercial banana producers, with research into the disorder dating back to the early 1970s in Australia. Much of the research was undertaken in Far North Queensland over 30 years ago by Jeff Daniells and other state government researchers at the time and has provided some interesting insights into what has proved to be a complex disorder.
What we know:
Maturity bronzing is not caused by a disease or insect but is a more complex physiological disorder due to certain environmental conditions.


The disorder blemishes the peel of banana fruit close to maturity and appears as bronze-red/brown streaks or blotches usually on the outer curved surface of the fruit and is more prominent in the top hands (Figure 1). The blemish can first appear when a bunch is three-quarters to full maturity stage and worsens as the fruit continues to fill. The damage is to the peel only and does not affect the yield and eating quality of the fruit. In severe cases, it can cause corking/cracking of the peel. Its appearance makes the fruit unmarketable and can account for significant losses for growers.
The disorder is associated with periods of heavy rainfall, high humidity and overcast weather conditions leading up to harvest and is therefore worse at certain times of the year. In Far North Queensland the disorder is usually more prevalent in the latter half of the wet season from March to June. Water stress at the time of bunch emergence has been shown to increase the severity of maturity bronzing.
Research by Dr Michelle Williams from the University of Sydney has shown that the high growth rates in the wet season lead to the stretching of the epidermis (outer surface of fruit peel) which exceeds its elastic limit, leading to cracks and cell disruption in the peel surface. Disruption of the cells causes the release of the enzyme called polyphenol oxidase. Oxidation of this enzyme leads to the production of melanin, which results in the bronze-red/brown markings within the peel. It is the same process in many fruits; for instance, when you cut open an apple and get brown discolouration of the cut surface.
Dr Williams’ research also found low levels of calcium in the fruit peel and low cell number in the peel epidermis have been linked to the disorder. They also found low calcium levels present in fruit suffering from water stress near bunch emergence. This stressed fruit had more severe maturity bronzing. Subsequent trials looking to increase calcium in the fruit peel to lessen maturity bronzing were unsuccessful.
There have been several trials looking into the effects of different agronomic practices on the disorder.
Trials looking into the effect of bunch covering found that normal bunch covering does not worsen the disorder but, the disorder is made worse by fruit from sealed bunch covers.
Both bunch trimming and de-belling (removal of the male bud) increase the severity of the disorder.
It is possible to reduce the severity of the disorder by reducing the leaf number to seven or less from bunch emergence. However, this is counterproductive as bunch weight and fruit green life are reduced at the same time.
How to manage and reduce the impact of maturity bronzing:
• Maintain good soil moisture levels, particularly in the period within 2 weeks of bunch emergence. The critical period is October to January, special attention should be paid to irrigation during this time. A high moisture level should be maintained during bunch emergence.
• Maintain even growth in the plant and the bunch, particularly from 2-3 weeks prior to bunch emergence up to harvest.
• Depending on market specifications, bunches can be harvested early before the disorder becomes severe. Blemished fruit losses are minimised but there is a trade-off with lower bunch weight. For every week that a bunch is harvested earlier, 7–10 % in bunch weight is lost.
• Improve drainage and light within your paddock to ensure bunches don’t take so long to reach a harvestable grade. Waterlogging can be minimised by mounding rows and the construction of deep drains on alluvial soils and light penetration can be improved by planting at moderate densities.
• Ensure bunch covers are not too long and not prone to sealing at the bottom of the bunch. If this occurs maturity bronzing will worsen.
If you would like more detailed information on past research into maturity bronzing, please contact the Better Bananas team at betterbananas@daf.qld.gov.au.
This information has been compiled as part of the National Banana Development and Extension Program (BA19004). This project has been funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Richard Piper
Richard Piper
A career exploring the big world of tiny things
Richard is the man to talk to if you want information on insects in Bananas. Richard is a familiar face to many banana growers in Far North Queensland, having worked in the industry both in a private and government capacity for over 30 years.
Over the past 5 years, Richard has worked as an entomologist with the Department of Agriculture and Fisheries (DAF) at South Johnstone. His work has encompassed screening of new chemistry for the control of bunch pests. These include synthetic as well as biological products such as fungi, bacteria and beneficial nematodes and botanical chemistries. Currently, he is continuing research on bunch pest management and spider mites, with a focus on integrated

Richard Piper
Entomologist
Department of Agriculture and Fisheries
Centre for Wet Tropics Agriculture
South Johnstone
pest and disease management (IPDM) practices using a combination of existing chemistry with softer biological options.
Richard’s expertise is not only limited to bananas. He is often out in the field talking with growers about insect related problems in other crops grown in the wet tropics.
It’s not all about bugs though. The interaction between grower, industry and colleagues is what Richard enjoys most about his work. He is excited about the future in gaining a better understanding of the relationship between insects and plants. Richard considers finding biological options for control of banana bunch pests as an important part of his role, seeing an increasing need for softer alternatives for future management strategies.
Richard was born and raised at Manly on Moreton Bay and completed his early schooling there. He attended the University of Queensland, where he completed a science degree, majoring in entomology and botany and then completed his honours in entomology. He also completed a post graduate diploma at Gatton College in plant protection.
In 1984 he moved to Far North Queensland to take up a position working as a medical entomologist with Queensland Department of Health on the Dengue Program for four years, then took work with the Australian Army’s Malaria Research Unit for four years where he was based at Cowley Beach and employed as a mosquito collector. In 1990 he started work with the Department of Primary Industries on an integrated pest management (IPM) project which he did for four years before starting his own business, assisting growers to monitor and manage pest insects and other problems in the many crops in Far North Queensland. Twenty seven years later he returned to DAF at South Johnstone taking up the role of entomologist in 2017.
Richard’s favourite way of eating bananas is by mashing a banana, preferably lady finger, on toast and sprinkling it with brown sugar.
In his spare time, Richard likes to tend his extensive garden of tropical fruit and vegetables.
Sarah Williams
Sarah Williams
Bringing passion to bananas
Sarah Williams is a recent addition to the National Banana Development and Extension team. Having moved to the Cairns region, Sarah has taken up the role with the Department of Agriculture & Fisheries and is based at South Johnstone. Sarah will be working to assist growers by shaping research to address their concerns. Currently, she is focusing on determining methods to improve bunch pest management practices to reduce fruit damage, waste, and raise the bottom line for growers.

Sarah Williams
Development Horticulturalist
Department of Agriculture and Fisheries
Centre for Wet Tropics Agriculture, South Johnstone, Qld
Completing her university degree in Environmental Science at the University of Technology Sydney, Sarah spent her final honours year focusing on using natural rooting hormones from plant extracts to improve planting establishment, to reduce dependencies on fertilisers. After finishing university, Sarah knew that she wanted to work to bring science to the people, equipping them to make decisions with the most recent research. Moving from NSW to Ayr, Sarah began her career in extension working at an independent agronomy firm with the sugar industry, working on projects that focused on precision agronomy, herbicide efficiency usage and alternative fallow cropping. Here, Sarah discovered her love for working with local growers and helping them get the best crop possible.
We asked Sarah what she’s most excited about in her new role ‘ I’m really excited to provide the industry with research that’s relevant, practical, and address’ growers’ concerns and limitations within the industry.’
Outside of work, Sarah enjoys hiking, camping and playing with her dog. Her favourite banana recipe is rum-soaked BBQed bananas.
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 15
- Next Page »