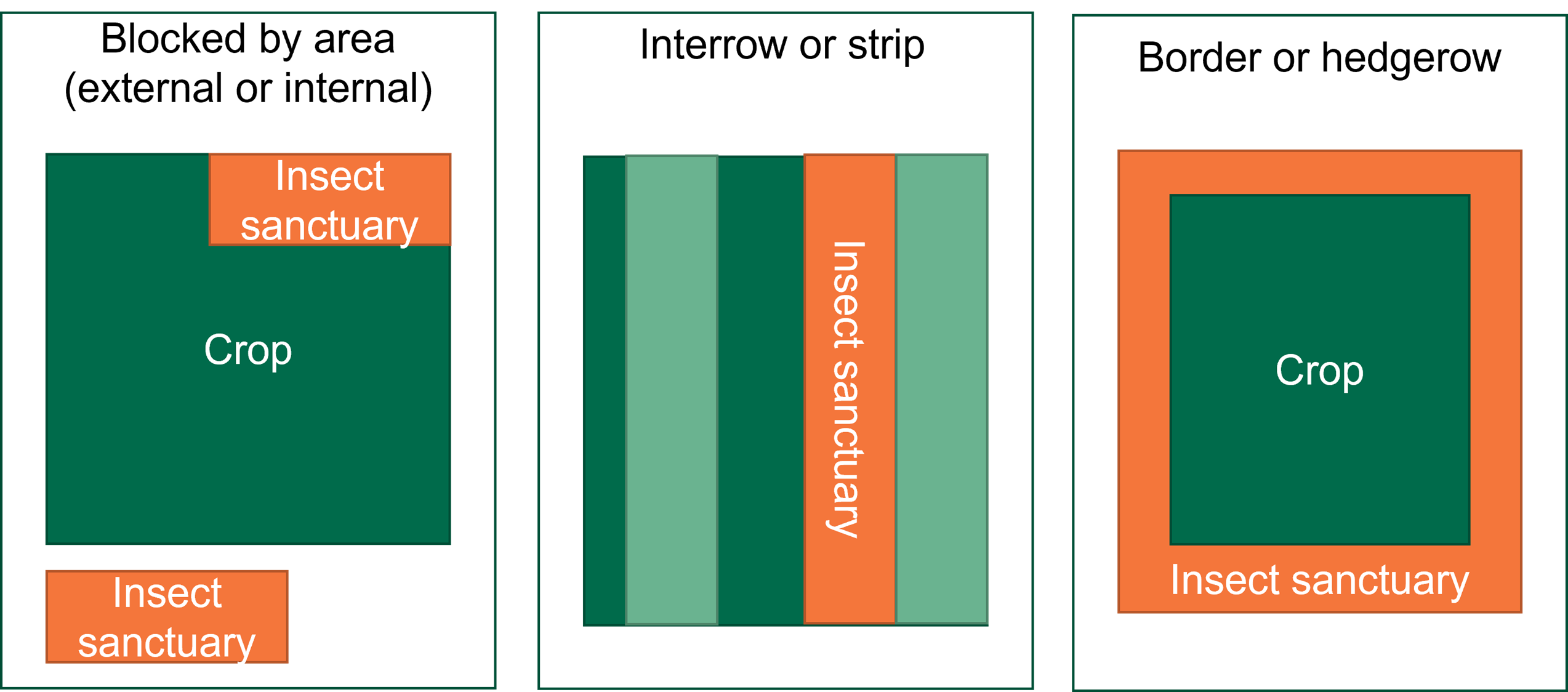
Yellow Sigatoka screening – agronomic evaluation trial (September 2018)
Katie Robertson, Jeff Daniells, Carole Wright and David East, Queensland DAF (Dec 2022)
Four CIRAD hybrids demonstrated good resistance to yellow Sigatoka, but they did not measure up on their other agronomic characteristics. Seventeen Cavendish lines were assessed for resistance, but non were any better than Williams. All were rated as very susceptible to yellow Sigatoka.
Yellow Sigatoka, also known as Sigatoka leaf spot, is the major leaf disease affecting the north Queensland banana industry and is caused by the fungus Pseudocercospora musae. Most of the bananas grown are of the Cavendish type (mainly Williams), as well as a small amount of Lady Finger, both of which are very susceptible to the disease. The annual cost of controlling leaf disease in the north Queensland banana industry is estimated to be in excess of $25 million. The disease is particularly difficult to control under hot, wet conditions and an integrated disease management program involving both cultural and chemical measures is required for effective control. Fungicides are usually aerially applied to the leaf canopy at regular intervals throughout the year and represent the major ‘pesticide’ applied in the production of bananas in north Queensland.

It would be a great advantage to the industry if a commercially viable variety possessed disease resistance. The cost of production could be significantly reduced, as well as the industry’s overall pesticide input and any associated environmental impacts – perceived or otherwise.
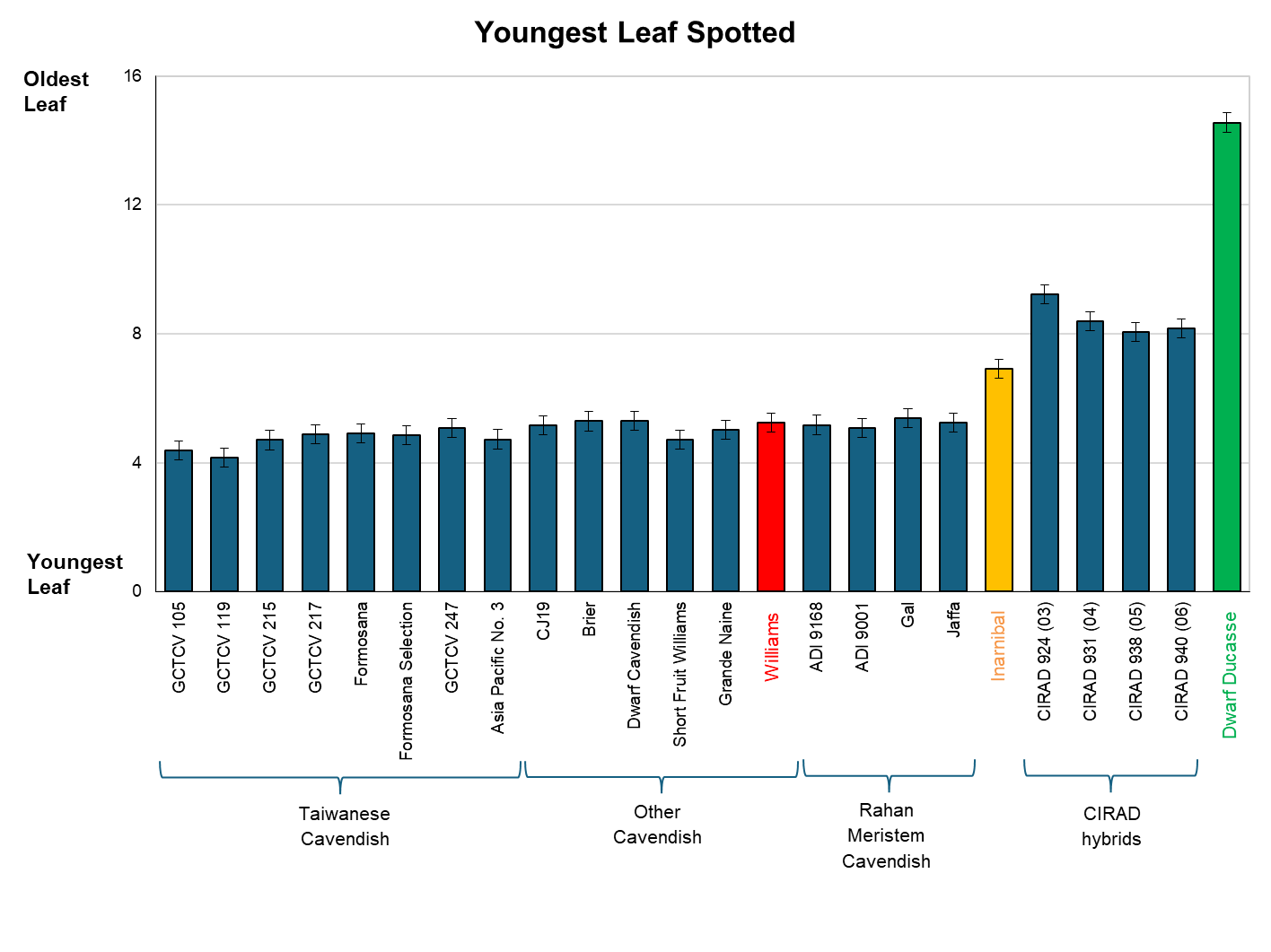
As part of the project ‘Improved plant protection for the banana industry’ (BA16001), 24 varieties were screened for resistance to Sigatoka leaf spot at South Johnstone Research Facility during the 2022 wet season. The same block of bananas where the plant and ratoon crop agronomic evaluations reported previously (see here), was used for the disease screening trial. After the final ratoon crop, fungicide applications for leaf disease control ceased. The block was then nurse-suckered to synchronise development so that leaf spot could be rated on plants prior to bunching during the wet season in 2022. Counting from the first fully unfolded leaf down, the youngest leaf with 10 or more mature lesions (youngest leaf spotted – YLS) was recorded and the youngest leaf with 33 per cent necrosis of the lamina (YL33). The total number of functional leaves (TFL) was recorded if no leaf spot symptoms were present. Ratings were done on three separate occasions in the last week of March, April, and May 2022, respectively. For simplicity, just the YLS or TFL results averaged over the three rating occasions are presented here.
The new varieties have been given an overall disease reaction rating relative to three reference varieties that have had their susceptibility/resistance categorised in previous studies. All the Cavendish varieties were very susceptible and their YLS values were not significantly different to Williams, except for two of the TR4 resistant selections from Taiwan (GCTCV 105 and GCTCV 119) which had slightly lower YLS values. Our intermediate reference variety, Inarnibal, had on average two more leaves present with less than 10 mature lesions compared to the Cavendish varieties.
The CIRAD hybrids exhibited no leaf spot symptoms but only had 8 – 9 upright leaves on average. Nevertheless, they were considered highly resistant along with Dwarf Ducasse – the highly resistant reference variety.


The CIRAD hybrids certainly have leaves with good resistance to yellow Sigatoka, but those same leaves are relatively brittle and are prone to snapping, thus reducing the functional leaf area. Additionally, their cumulative yields in the preceding crops were 28-36% lower than that of Williams while also being 15-30% taller. CIRAD 924 and 938 had an acceptable taste and have also shown to be resistant to TR4 in the NT (along with CIRAD 931) but when all their characteristics are weighed up, there seems to be little commercial prospect for these varieties despite their good Sigatoka leaf spot resistance.
More information
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.