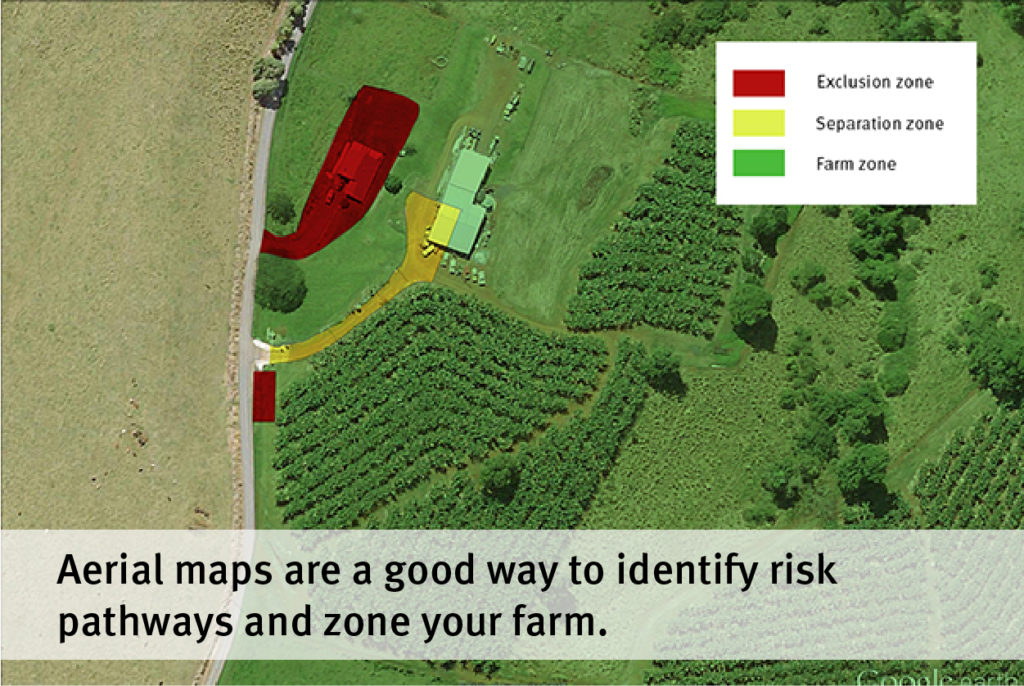
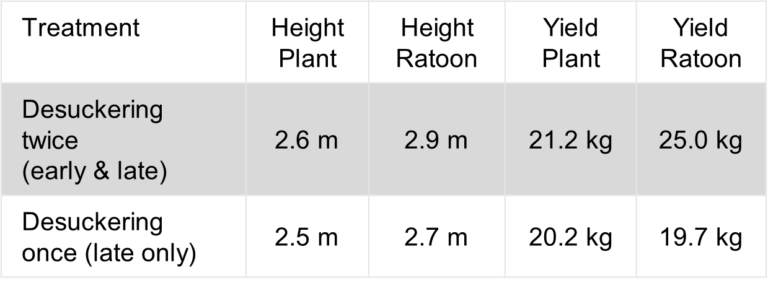
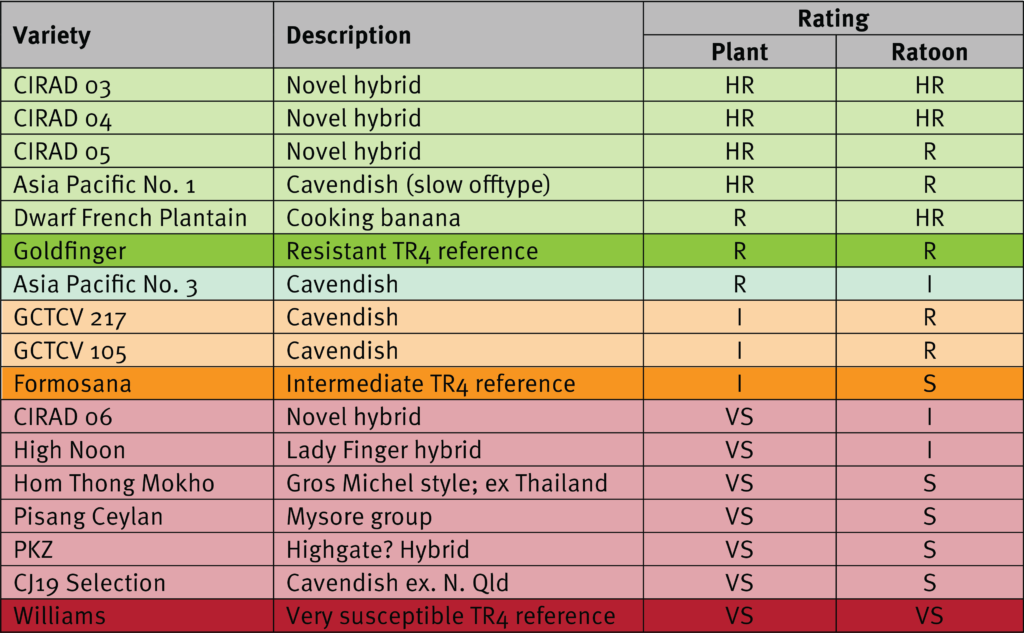
Generally, the focus of the NT screening trials is to identify resistant varieties, especially those that display similar or better resistance than Formosana, as it is used as the benchmark for the lowest acceptable level of resistance. In this trial eight varieties demonstrated better resistance than Formosana, which include Cavendish and CIRAD lines.
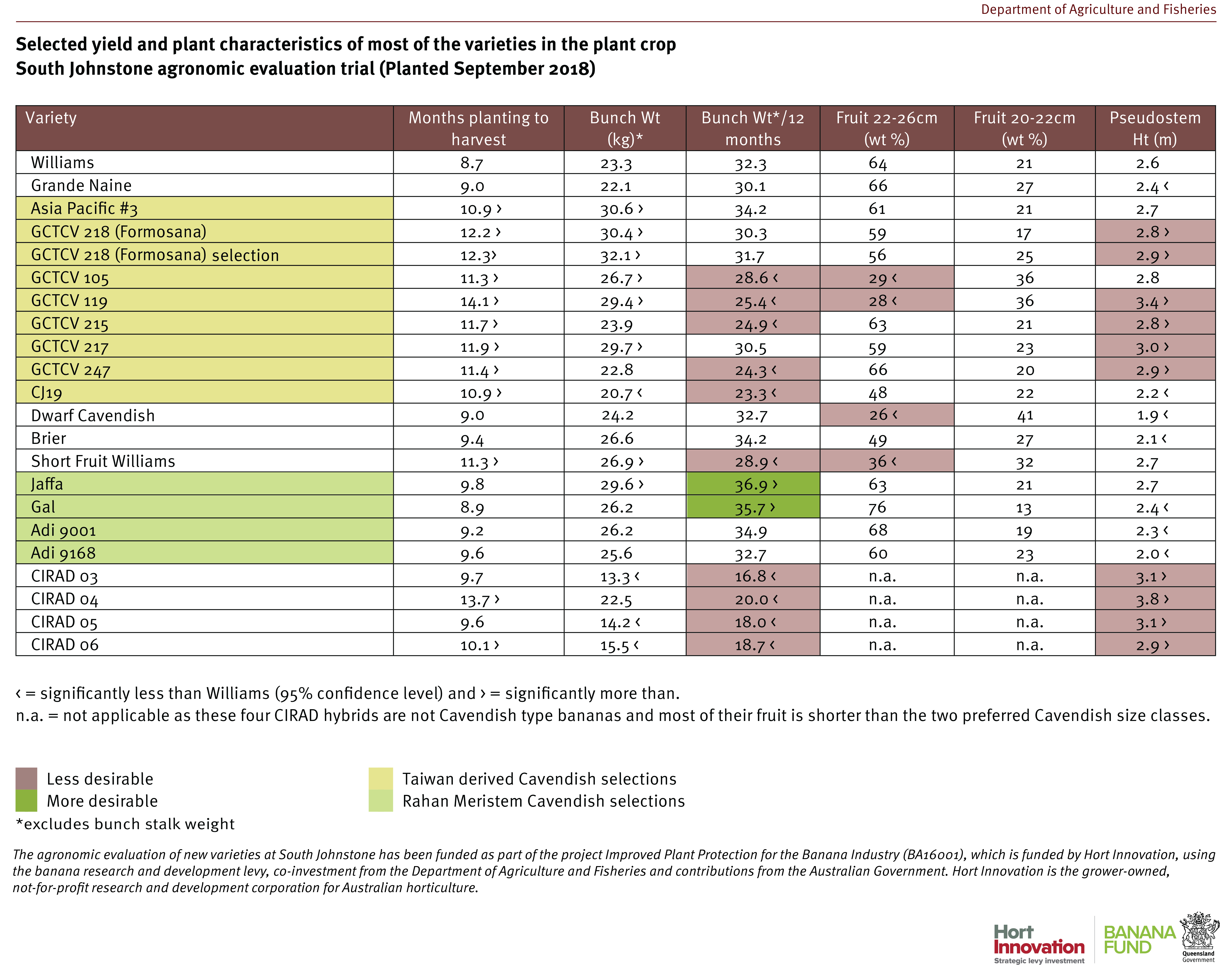
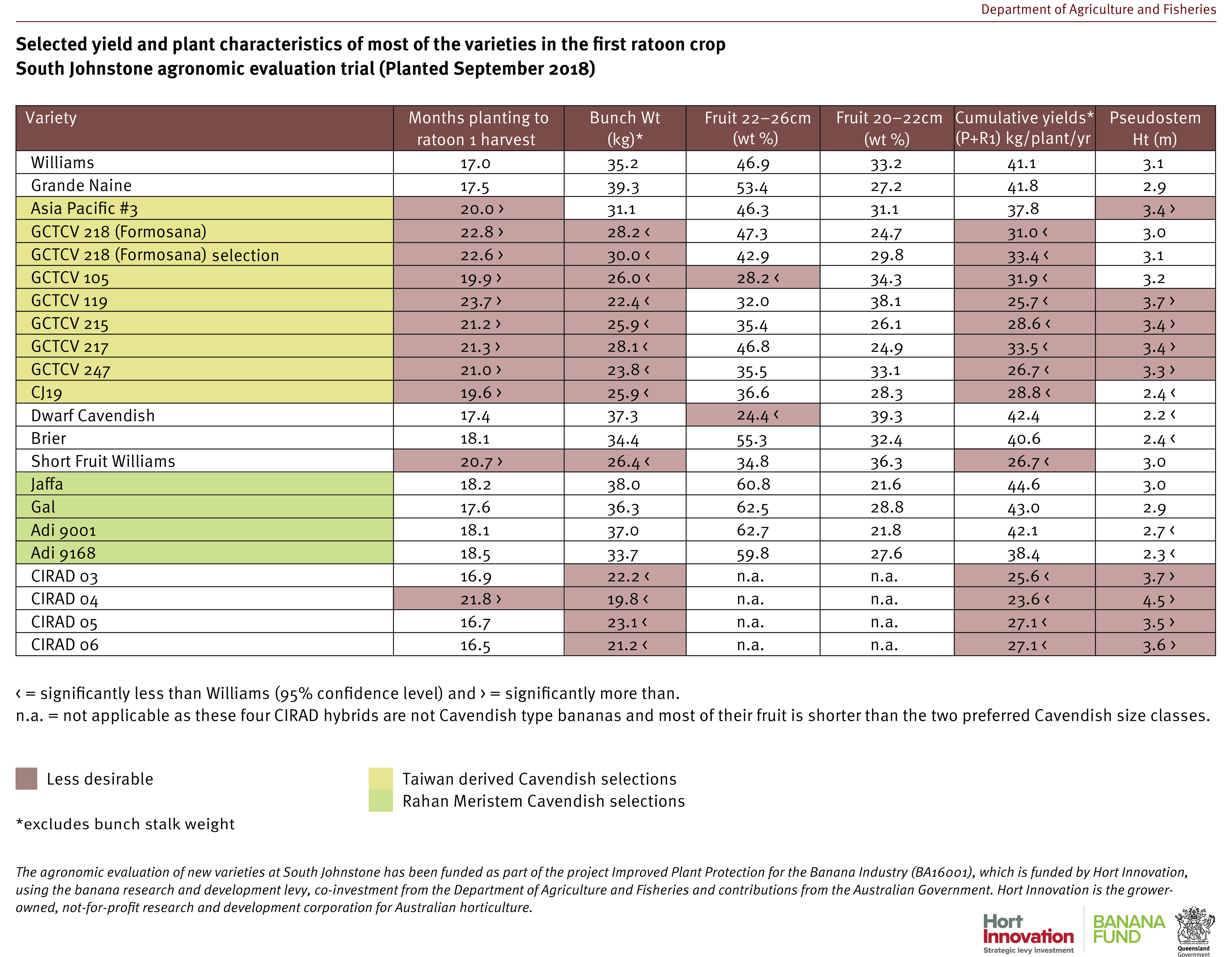
The Cavendish selections Asia Pacific No. 3 and GCTCV 217 both performed better than Formosana against TR4 and have also performed fairly well agronomically in north Queensland, so they are being considered for on-farm pre-commercialisation trials in 2022. There were two other Cavendish with intermediate or resistant reactions: the Asia Pacific No. 1 plants were all tissue culture offtypes (very slow and low yielding), whilst the fruit of GCTCV 105 was too short in South Johnstone trials, with significantly less fruit in the currently required size range for market.
The continued good performance of Dwarf French Plantain against TR4 is encouraging for the few commercial producers of this type of niche variety. The CIRAD lines 03, 04 and 05 all had outstanding resistance against TR4 across the 2 crops which is very encouraging for the French breeding program. However, growers must understand that these are not Cavendish and so the fruit they produce does not slot readily into the current market requirements. The plants are taller making them more difficult to manage and subject to greater wind damage.
Although apparent ‘recovery’ occurred in some varieties within the first ratoon crop, a degree of caution is required when interpreting these results, particularly in the case of CIRAD 06 and High Noon. Although the results of those two varieties are very interesting, this would need to be investigated further to determine how repeatable such a recovery is, and whether indeed, it would continue into later ratoon crops.