Banana scab moth Nacoleia octasema
General information
Occurrence
Banana scab moth is present throughout the year but is favoured by moist and warm conditions, hence the greatest potential for damage is during the wet season. Bunches that emerge from December through to the end of May are most at risk of severe fruit damage. The cooler and drier winter months are relatively free of banana scab moth damage. However, damage can occur if unseasonal rain occurs at this time. Research has shown adult moths do not mate or produce eggs under low humidity and dry conditions.
Description and lifecycle
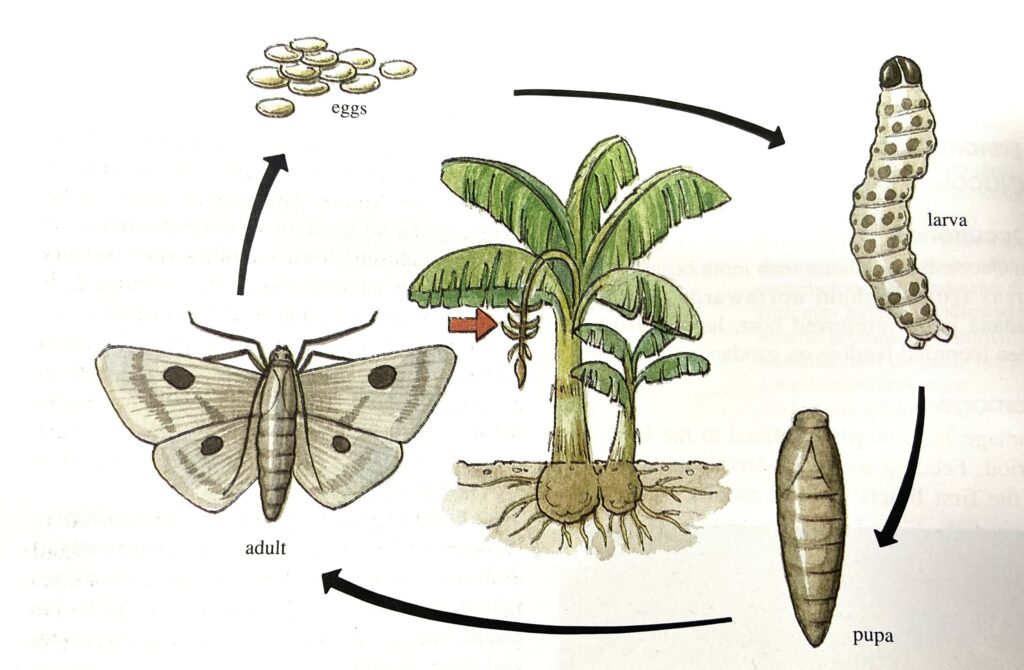
The tiny (1.2-1.5mm) transparent or yellow flattened eggs are laid in clusters (of up to 30 eggs) that resemble miniature overlapping fish scales. These egg clusters are very difficult to locate because of their small size and the fact that they are laid near the throat of the plant. The eggs are usually laid on the emerging bunch and the surrounding leaves, but eggs have occasionally been found on the pseudostem below the new bunch. Larvae (caterpillars) are pink to brown in colour and range in length from 1.5mm when first hatched to about 25mm when fully developed. If disturbed the larvae wiggle violently and drop on silken threads to avoid predation. When larvae are fully mature they generally pupate in the trash at the base of plants or beneath dry leaf sheaths.
The adult moths are difficult to find due to their small size (22mm wingspan), the fact they hide during the day and their dull brown/grey colouration. Adults are most active at dusk when mating and egg laying occurs. Adults do not appear to be attracted to lights, unlike other moth species. The total lifecycle from egg to mature adult takes around 25-32 days.

Damage
The banana scab moth is a severe pest of bananas and can cause up to 100% damage to the bunch if left uncontrolled.
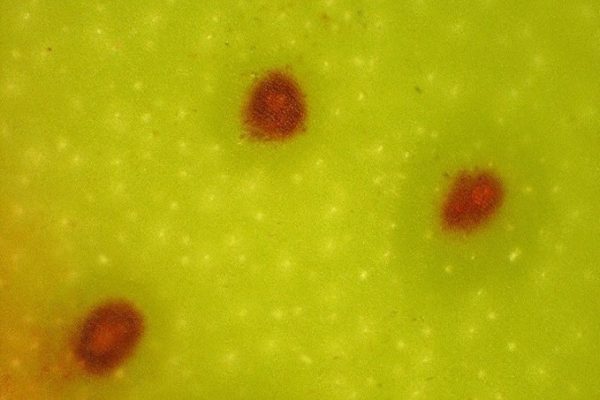
Feeding by young larvae starts as soon as the first bracts lift and usually increases in severity as the larvae grow and move progressively down the bunch as subsequent bracts open. The feeding causes cracking and scarring to the fruit skin, while severe cases can cause disfigurement of fruit as the fingers enlarge. Damage is usually only superficial, where affected fruit is downgraded or deemed unsuitable for the market.
Damage is usually confined to the outer curve of the fingers (the area nearest to the bunch stalk) but, in more severe cases, damage can extend to the stalk, areas between touching fingers, or even extend to cover the whole fruit surface.




Larvae of banana scab moth also consume foliage and can damage plants where a bunch is absent. This leaf damage is worse in varieties such as Lady Finger and Ducasse and is generally not a problem in Cavendish.
For more information contact:
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
13 25 23 or email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been updated as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.