New test helps product screening for Chalara management
New test helps product screening for Chalara management
Multiple fungal organisms are known to cause Crown end rot (CER) in bananas. The following research is focused on the more serious form of CER commonly known as Chalara where the rot extends into the fruit (caused by Thielaviopsis musarum). Disease symptoms are typically observed in the supply chain during cooler periods of the year (winter). Chalara is sporadic in occurrence, making it difficult to conduct research trials with the disease. Department of Agriculture and Fisheries researchers have now developed an inoculation technique that mimics the development of Chalara in the supply chain, enabling researchers to screen and evaluate alternative management options.
There are two post-harvest fungicides currently registered for use in Australia to help manage CER. Although these treatments are effective against the fungi that cause CER, growers have expressed a need for non-chemical options for managing the disease, particularly those with organic status.
The inoculation technique has now been used to determine efficacy of the currently registered fungicides, alternative fungicides and biological products.
Results
Overall, the inoculation technique developed is rapid and reliable and the results are reproducible. Even though the technique was specific for Chalara (T. musarum), crown mould assessments were also obtained. Ideally a successful test product should have efficacy against T. musarum and the range of fungi that cause crown mould.
Prior to conducting this research there was only anecdotal evidence that the current registered projects had efficacy against T. musarum, but this has now been confirmed, with both Tecto® and Protak® effective in halting the development of Chalara. Results also showed that some biological products are capable of managing Chalara and reducing levels of crown mould.
Participating companies have been supplied the results for their products. They can use the results to support registration applications and/or determine which products are worth investing in further trials. It is hoped this work will lead to product registration adding alternative management options for growers.



Remember...
Before using any chemicals, always check the current registration status and read the product label. Label and permit details can be accessed via APVMA website: www.apvma.gov.au
This work was undertaken as part of the ‘Enhancing the outcomes of BA13011-Crown end rot investigations’ funded as part of Department of Agriculture and Fisheries’ Horticulture and Forestry Science development funding.

Meet a researcher – David East
David East
Unravelling the mysteries of plant diseases
Agriculture has always been a big part of David’s life. Growing up on a mixed grazing property just north of Orange in the Central Tablelands of NSW, he went on to study Systems Agriculture at The University of Western Sydney. After finishing Uni, David worked in the cotton industry in Western NSW before taking an opportunity to manage a large forestry nursery. It was David’s career in the forestry sector that led him to move to Tully with his young family, managing propagation for a large forestry company. In 2014, David decided on a different career path, taking on a technical laboratory position with the Department of Agriculture and Fisheries based at South Johnstone.
Since joining the Department David has forged a successful career in plant pathology. His current research work involves a yellow Sigatoka leaf spot trial, evaluating the effectiveness of new chemistry and ‘softer options’ for control. In addition to this work, he is also busy providing general diagnostics for the banana industry.

David East
Plant Pathologist
Department of Agriculture and Fisheries
Centre for Wet Tropics Agriculture, South Johnstone, Qld
We asked David what is the most exciting part of his research. ‘The most exciting part of my current research is exploring new solutions to perennial problems within the banana industry. The most enjoyable part of my job is diagnostics. Each sample is its own little mystery. It is incredibly satisfying to identify the problem, explore the factors that led up to issue, and to advise ways to avoid it happening again’.
Outside of the lab David enjoys gardening, spending time with his family, playing guitar and fishing. His favourite banana recipe is banana bread, which he often enjoys for smoko.
Banana bunch cover trial
The colour of your bunch covers may help control banana rust thrips
Banana rust thrips continue to be a significant pest for banana growers with levels of damage increasing in recent years. The thrips cause damage by feeding on the skin of immature banana fruit which causes reddish-brown marks. Growers are reporting that even fruit with low levels of damage are not meeting market specifications.
So what role does the colour of bunch covers play in rust thrips damage? Interest amongst researchers was sparked after previous work had shown that rust thrips respond differently to different coloured sticky traps. This prompted researchers to have a look into the effect that different coloured bunch covers have on thrips damage. The aim is to find non-chemical control methods as part of an Integrated Pest Management (IPM) strategy.



Initial trial results are encouraging and do show a difference in the level of damage caused by thrips depending on the colour of the bunch covers used. In this trial no chemical treatment was applied to the bunch after bell injection and the bunch cover was applied as per commercial timing. Orange, yellow and purple bunch covers showed damage above commercially acceptable levels in this scenario. The best performer was a paper bunch cover with a polyethylene ‘cloth’ liner. Light blue and white also produced similar low levels of damage compared with some other colours.
Finger length, colour and bloom were also assessed with results indicating that bag colour has no significant effect on these fruit quality attributes.
Based on the initial trial results growers should consider using bunch cover colours that have a low thrips damage rating. This coupled with standard insecticide treatments applied at bunch covering should provide the best level of control. Further work is underway to expand these results by testing new colour and liner combinations. Recommendations of the latest trial will be available to growers in the coming months.
More information
This research is funded as part of the Improved Plant Protection for the Banana Industry Program (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Banana bunch cover trial information
Effects of using different coloured bunch covers on banana rust thrips damage
About the trial
The trial looked into the effect that different coloured bunch covers have on Banana rust thrips damage. The aim is to find non-chemical control methods as part of an Integrated Pest Management (IPM) strategy.
The only chemical treatment applied in the trial was omethoate injected at bell emergence to ensure researchers were only measuring the influence that bunch covers had on rust thrips damage. This meant that there was no damage to the fruit before the bunch covers were put on.
As per commercial practice, bunch covers were placed on bunches when all the bracts had fallen off, approximately a fortnight after bunch emergence. At the time of bagging, the bell and the false hand plus one were removed.
Rust thrips damage was assessed at harvest by examining the surface of 5 central fingers from the top, middle and bottom hands of the bunch.
The presence and extent of damage on the fingers assessed was recorded using a scale of 0 to 4. Rating 1 is defined by a faint halo and is considered the maximum rating for a commercially acceptable level of damage.

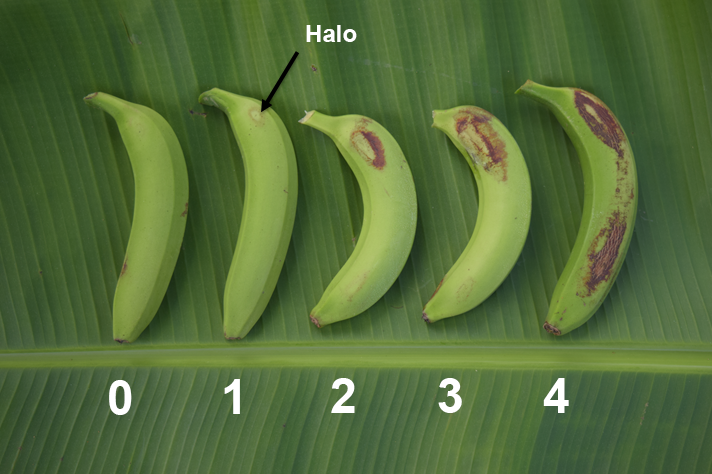
Measuring the effect that bunch cover colours had on other fruit quality characteristics was also important to ensure they didn’t have a negative impact. The following fruit quality attributes were therefore assessed at harvest.
- Finger length and diameter
- Colour (Hue, chroma and lightness) of the peel at fruit colour stage 1 and 6
- Bloom (the ‘lustre’ or ‘shine’ of the fruit) was assessed photographically to assess peel reflectance
* Note – Omethoate was registered at the time the trial was conducted and has since been deregistered. Before using any chemicals always check the current registration status and read the product label. Label and permit details can be accessed via the APVMA website, www.apvma.gov.au.
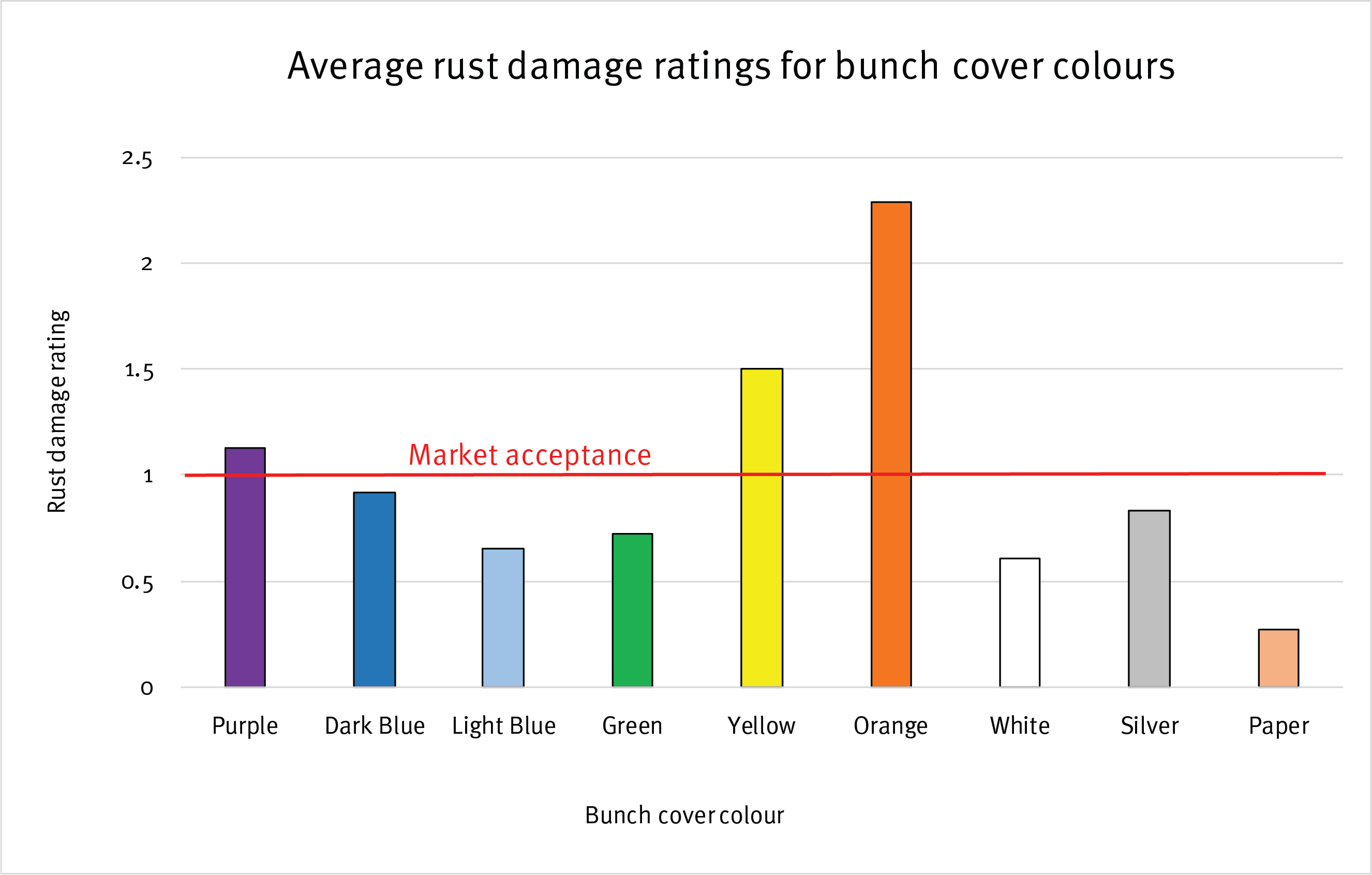
Results from the initial trial indicate that colours do play a role in level of rust thrips damage
The initial trial results showed a lot of variation in the damage caused by banana rust thrips with respect to different bunch cover colours.
Orange, yellow and purple bunch covers had levels of damage above commercially acceptable levels. Statistically, orange had a significantly higher level of damage compared to all other covers.
The best performer was a paper bunch cover with a polyethylene ‘cloth’ liner. This was the only bunch cover that had a liner in the initial trial. Therefore a new trial is looking at the impact of liners in combination with different bunch cover colours. Light blue and white produced similar low levels of damage as the paper bag.
Results also indicate that bunch cover colours have no statistically significant effect on finger length, diameter, colour and bloom.
Growers should consider using bunch cover colours with low thrips damage rating
Based on the initial trial results, growers should consider using bunch cover colours that scored a low thrips damage rating. Further work is underway to expand these results by testing new colour and liner combinations. Results of the latest trial will be available to growers in the coming months.

If you would like further information on this trial, contact the Better Bananas team.
This research is funded as part of the Improved Plant Protection for the Banana Industry Program (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Katelyn Ferro
Katelyn Robertson
After finishing an Ag Science degree at the University of Queensland’s Gatton campus, Katie made the move to Far North Queensland, accepting a research horticulturist role at South Johnstone with the Department of Agriculture and Fisheries. Over the past three years, working with the banana research and development team, Katie’s main focus has been research into Panama disease.
Katie is currently working in banana variety research, assessing the post-harvest characteristics of fruit from the Goldfinger mutagenesis trial. This work is aimed at finding a commercially viable banana variety that has resistance to Panama disease tropical race 4.
We asked Katie what is the most exciting part of her research and what she enjoys the most. “I’ve come to realise how unique banana plants are compared to most other crops and, although often frustrating, I like the challenge this presents and the problem solving required to develop research methods tailored to its individuality. I really enjoy working on impactful projects where the outcome of the research could benefit Australian banana growers.”
Katie’s childhood was spent in Delaneys Creek in the Sunshine Coast hinterland, but lived in Brisbane for all her schooling years. Her love for the outdoors sees her hiking North Queensland’s beautiful national parks. Katie is also keen to learn new skills and is currently learning how to play electric guitar. Her favourite banana recipe is Banana ice-cream, made from frozen banana that is blended until creamy.

Research Horticulturist
Department of Agriculture and Fisheries
Centre for Wet Tropics Agriculture, South Johnstone, Qld
Agronomic evaluation of new varieties – South Johnstone
Latest update...
The first ratoon crop is now completed in the variety trial and the results are encouraging with:
- The TBRI Cavendish selection Asia Pacific #3 showing comparable yields and fruit length to Williams over the two crop cycles, combined with Panama disease TR4 resistance much better than Formosana in the NT trials.
- Continued good performance of the four Cavendish selections from Rahan Meristem with yields and finger length equivalent to Williams, with at least two of the selections being significantly shorter in stature.
- The Dwarf Cavendish selection Brier, from the Canary Islands, having yields and fruit length equivalent to Williams, while being significantly shorter in stature.
Click here for more information on first ratoon observations and results
About the trial
Growers are keeping a keen eye on the 32 varieties included in the latest agronomic evaluation at South Johnstone. This is the first step at looking at new introductions that may have commercial potential for the Australian banana industry.
This research forms a significant part of the project Improved Plant Protection for the Banana Industry (BA16001), looking at the agronomic traits as well as pest and disease tolerance of imported varieties. This project provides for 3 variety assessment trials across Australia at Alstonville (previously Duranbah NSW), South Johnstone (Qld) and Coastal Plains (NT), assessing resistance to Panama disease Race 1 and TR4, agronomic performance, cold tolerance and yellow Sigatoka resistance.
Several of the varieties included in the current South Johnstone trial are also being screened in the Northern Territory to determine or confirm resistance to TR4.
Varieties were planted in September 2018 and assessment of agronomic traits will be collected over three crop cycles and a yellow Sigatoka leaf spot screening in the fourth cycle. Several new varieties that have shown resistance to TR4 overseas are included in the evaluation.
With an agreement now in place with the Taiwan Banana Research Institute, it will be possible to progress some of the better performing Taiwanese varieties to on-farm trials. The ability to grow these varieties as part of on-farm trials will allow for a greater number of plants than what is possible at South Johnstone Research Facility.



Varieties being evaluated
- A suite of Taiwanese selections of Cavendish present in Australia. Also included is a selection made in Australia from a former introduction.
- Agro-biotechnology company Rahan Meristem imported four of their elite Cavendish selections into Australia from Israel— Gal, Jaffa and two selections of Adi. The main features include reduced plant stature and large well-structured bunches. These selections are proving popular in various export production zones around the globe. However, these selections are not claimed to have any resistance to Panama disease tropical race 4 (TR4). North Queensland producers that have seen them growing overseas have been keen to see them evaluated by the Department of Agriculture and Fisheries (DAF) for some time. Rahan Meristem own these varieties and have agreed that results from our evaluations can be made publicly available.
- Four hybrids from the breeding program of CIRAD in the French West Indies. Overseas these have shown resistance to leaf disease and Panama disease race 1. Three of these hybrids have shown good resistance to Panama disease TR4 in our Northern Territory trials completed last year.
- Two Cavendish selections from the Canary Islands. These selections of Dwarf Cavendish form the basis of their 400,000 t/yr. export industry to mainland Europe.
A list of varieties being evaluated is now available.
Observations and results
Observations and results are now available for both plant crop and first ratoon.
What's next?
Harvest of second ratoon bunches is now nearly completed. About 10% of the data plants were damaged in early March due to the strong winds brought on by the tropical low (which later developed into Cyclone Niran). Due to the development stage of the Taiwanese Cavendish varieties, these suffered the highest losses. Come November/December the next step will be to nurse sucker the block to synchronize development for leaf spot resistance assessment in the 2022 wet season.
In addition, a new trial was planted at South Johnstone in October 2020. We are evaluating some new varieties which have cleared quarantine since the present trial was established in 2018, along with some improved selections which have been identified in Australia.
Field walks of the trial have provided the opportunity for growers and industry stakeholders to view bunches hanging during the plant and first ratoon crops.

More information...
If you would like further information feel free to contact the Better Bananas team via email at betterbananas@daf.qld.gov.au.
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Images of Panama disease tropical race 4
Images of Panama disease tropical race 4 (TR4)





Images courtesy of Department of Primary Industries
Disinfectants
Disinfectants prove to be an integral part of on-farm biosecurity
Using disinfectants as part of your cleaning regime is vital to minimise the spread of Panama disease. Research shows that various disinfectant products are effective in killing fungal spores that cause Panama disease.
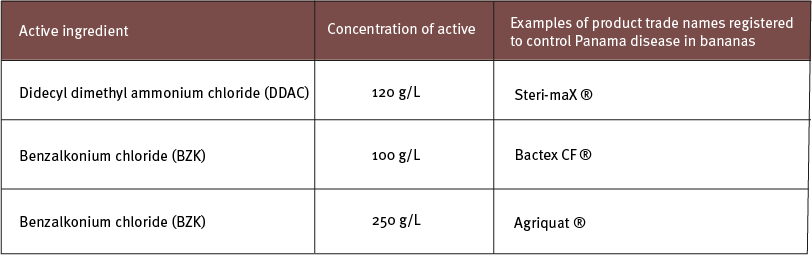
Products listed in the table below are examples of disinfectant products which were shown to be effective when applied at 1% solution.

Remember...
Before using any chemicals always check the current registration status and read the product label. A minor use permit is available for banana growers to use these products off-label (PER 86485) http://permits.apvma.gov.au/PER86485.pdf. Label and permit details can be accessed via APVMA website: www.apvma.gov.au
It is important to note that soil reduces the effectiveness of disinfectants. Different soil types have varying impacts on the effectiveness of the products. However, research has shown that once the equivalent of 1g of soil is present in 20mL of solution (1% product) then the effectiveness of products is compromised or reduced.
Investigations into DDAC products (e.g. Steri-maX®, Path-X™, Sporekill® made at 1% solution) has found that when there is no soil contamination, these products remain effective as a disinfectant after being exposed to sunlight, temperature and humidity for up to 12 months.
Quaternary ammonium compound test strips have shown to be an effective tool that measures the concentration of the active ingredient DDAC or BZK in disinfectant products. These easy-to-use test strips do not require dilution of the sample and are used by comparing the colour development on the test strip to the colour scale.
In summary:
- DDAC and BZK disinfectant products used at the correct concentration and as per label or permit specifications (contact times) do kill the fungal spores that cause Panama disease.
- It is important to remove all soil and organic matter before applying any disinfectant product and replace solutions if they become contaminated.
- Easy-to-use test strips can be used to regularly test solutions in footbaths, spray shuttles and wash-down facilities.
More info...
Remember: Always follow and adhere to product label rates and instructions. Label and permit details can be accessed via APVMA website: www.apvma.gov.au
This research was funded as part of project BA14013 Fusarium wilt Tropical Race 4 – Biosecurity and sustainable practices which was funded by Hort Innovation, using the Banana research and development levy, co-investment from the Queensland Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned not-for-profit research and development corporation for Australian Horticulture.



How can I test my QA disinfecting products?
Test strips can help determine the effectiveness of your disinfecting solution
Disinfectant products that contain the active ingredient didecyl dimethyl ammonium chloride (DDAC – e.g. Sporekill®, Steri-max® and Path-X™) or benzalkonium chloride (BZK – e.g. Bactex, Agriquat) have shown to kill fungal spores that cause Panama disease.
For these products to be effective, it is important they are mixed at a 1% solution. Our researchers have investigated a range of test strips to measure the concentration of the active ingredient of disinfectant products used in footbaths, spray shuttles and drive-through dips.
High level (0 – 1500ppm) or extra high level (0 – 10,000ppm) quaternary ammonium compound test strips are an easy method of testing the active ingredient DDAC or BZK by comparing the colour development on the test strip to a colour scale.
For your information...
1% solution of 120g/L DDAC (e.g. Steri-max®) equates to 1200ppm
1% solution of 100g/L BZK (e.g. Bactex) equates to 1000ppm
1% of solution of 250g/L BZK (e.g. Agriquat) equates to 2500ppm *
*(either dilute and use high level test strips or use extra high level (0 – 10 000ppm) test strips)

For best results, make up a 1% standard solution of the DDAC or BZK disinfectant product you are using. This will allow you to directly compare the exact colour of a 1% solution to the colour of the disinfectant sample you want to test.
Keep this solution in a sealed container for future use.
It is recommended...
It’s recommended that you dip your test strip into the 1% standard solution and the disinfectant sample (e.g. footbath) simultaneously, that way you can compare colour instantly.
1. Dip

Dip test strips into the 1% standard solution and your disinfectant sample and remove immediately.
2. Compare
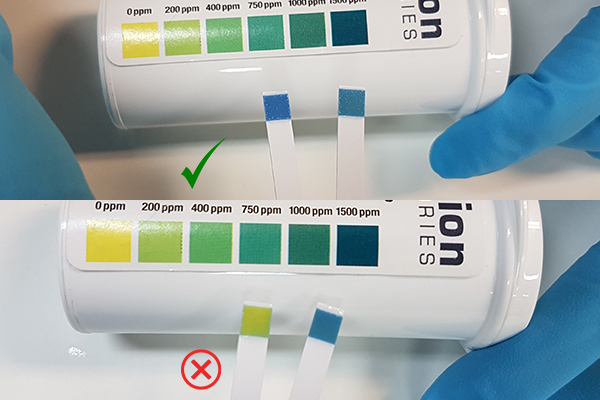
Immediately compare test strip to colour scale (maximum reading time of 5 seconds).
The colour of your disinfectant sample should be greater than or equal the 1% standard solution (as per image above with green tick).
In summary:
- Use test strips to regularly check the disinfectant solutions in your footbaths, spray shuttles and wash-down facilities to ensure they are at an effective concentration (1% solution).
- Ensure there is less than 5% soil in footbaths and wash-down facilities – that is equivalent to 1g of soil to 20mL of disinfectant solution (1% DDAC or BZK product).
- Be aware that soil with a high clay content may have the potential to influence test strip results.
- Different water sources do not appear to influence the test strip results.
- With no soil present in your disinfecting solution, DDAC products prepared to a 1% solution are still effective at managing spores that cause Panama disease.
For more information about this work or for details on where to purchase the test strips contact the better bananas team –betterbananas@daf.qld.gov.au or 13 25 23
Please note...
There may be factors beyond the scope of the research that has been undertaken using the quaternary ammonium compound test strips which have the potential to influence results.
This trial was funded as part of project BA14013 Fusarium wilt Tropical Race 4 – Biosecurity and sustainable practices which was funded by Hort Innovation, using the Banana research and development levy, co-investment from the Queensland Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned not-for-profit research and development corporation for Australian Horticulture.


- « Previous Page
- 1
- …
- 7
- 8
- 9
- 10
- 11
- …
- 15
- Next Page »







