Banana scab moth – monitoring and control
Banana scab moth – Nacoleia octasema
Monitoring and control options
Monitoring
Being proactive and regularly checking bunches or getting feedback from baggers can help growers discover infestations before the fruit reaches the packing shed. Although damage can’t be undone, identifying, and addressing problems (retraining or integrating alternative control options) sooner can save other bunches from impact.
The method for monitoring for banana scab moth is to inspect freshly emerged bunches (bract fall) for the presence of damage and/or larvae. Pay attention to the underside of the fingers in each hand (closest to the bunch stalk) and the cushion area. In very young bunches, lifting the developing hand away from the bunch stalk may be necessary to reveal any larvae and/or fresh damage.

Also, examine the base of the bunch stalk where the larvae enter the throat of the plant. Larvae can be detected by separating the base of the flag leaf and removing the bract that is attached to the stalk. Often a clear jelly-like substance or frass feeding waste, which appears to be associated only with banana scab moth feeding in bananas, is present at these sites. Monitoring known ‘hot spots’ such as rows adjacent to scrub or creek lines is also a good idea. Banana scab moth has been documented to use Pandanus spp. and Heliconia spp. as alternative host plants.
Managing banana scab moth
Treatment for banana scab moth should be performed year-round as damage results in immediate downgrading or rejection of fruit. Management of banana scab moth is particularly important if heavy bunching is anticipated and/or the forecast weather conditions are favourable (hot and wet).
Biological control
Banana scab moth can be controlled by application of Bacillus thuringiensis subsp. Kurstaki based biopesticides (also known as B.T.) that will not adversely affect other beneficial insects. Some insects (parasitic flies and wasps, ants, spiders and other predators) feed on banana scab moth caterpillars and provide some level of control.

Cultural control
Selecting followers of equal size which equates to synchronised bunch emergence over a block will ensure that the application of chemical control methods is more efficient.
Chemical control
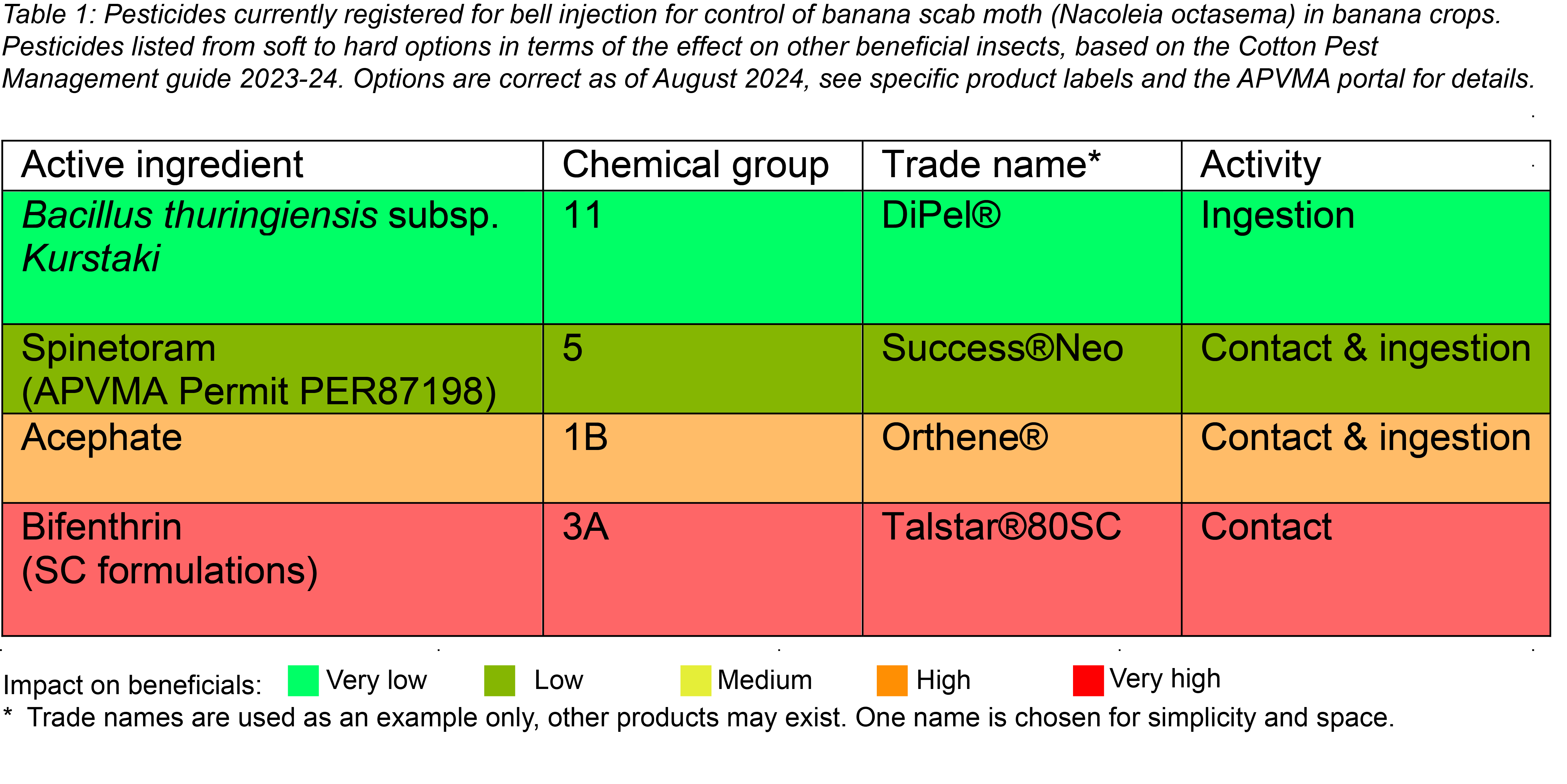
Bell injection is the preferred method of insecticide application to prevent banana scab moth damage. Bell injection is a targeted application of insecticide into the newly emerged bunch and the technique is unlikely to impact beneficial insects on other parts of the plant. The correct site for injection is approximately one third of the way down from the top of the upright, vertically positioned bell. Bells which are injected later than this (i.e., when horizontal) have an increased risk of insect damage.
Initial trial work completed by the Department of Agriculture and Fisheries (DAF) has also indicated that the volume of insecticide applied in bell injection is an important consideration. As insecticides require larger volumes to ensure good coverage, initial trial work showed that using 40mL volume wasn’t sufficient to get appropriate control at times of high pest pressure. It is recommended that growers use a 60mL volume at the specified label (or permit) rate for bell injection to provide adequate coverage.
Always check the APVMA website for current chemical registrations before use. Below are insecticides currently registered (August 2024) and permitted for bell injection to control banana scab moth.
For more information contact:
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
13 25 23 or email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been updated as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Banana Scab Moth – general information
Banana scab moth Nacoleia octasema
General information
Occurrence
Banana scab moth is present throughout the year but is favoured by moist and warm conditions, hence the greatest potential for damage is during the wet season. Bunches that emerge from December through to the end of May are most at risk of severe fruit damage. The cooler and drier winter months are relatively free of banana scab moth damage. However, damage can occur if unseasonal rain occurs at this time. Research has shown adult moths do not mate or produce eggs under low humidity and dry conditions.
Description and lifecycle
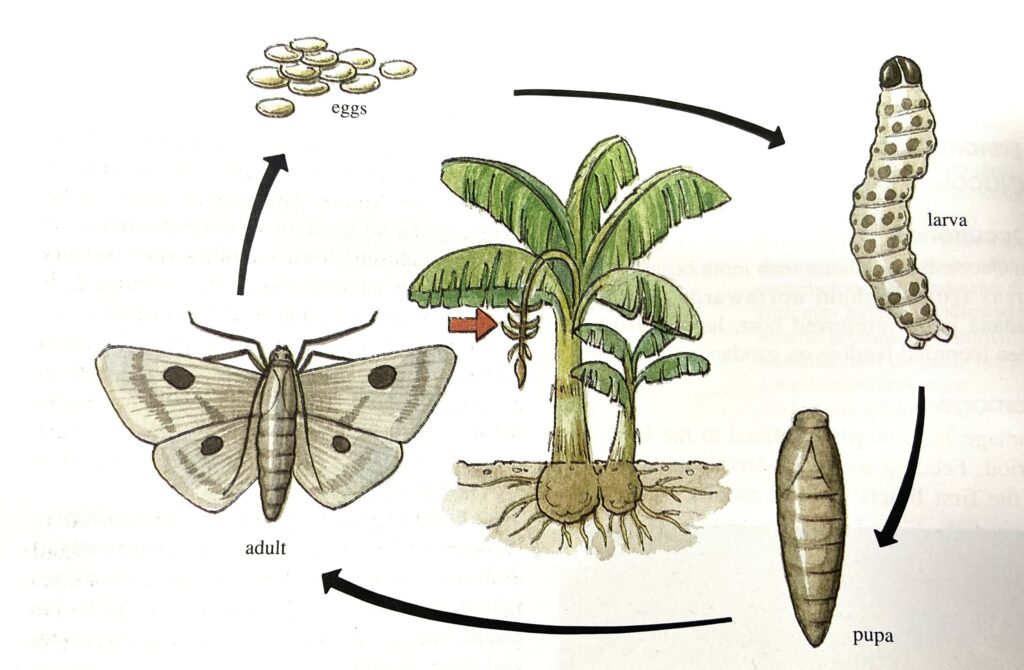
The tiny (1.2-1.5mm) transparent or yellow flattened eggs are laid in clusters (of up to 30 eggs) that resemble miniature overlapping fish scales. These egg clusters are very difficult to locate because of their small size and the fact that they are laid near the throat of the plant. The eggs are usually laid on the emerging bunch and the surrounding leaves, but eggs have occasionally been found on the pseudostem below the new bunch. Larvae (caterpillars) are pink to brown in colour and range in length from 1.5mm when first hatched to about 25mm when fully developed. If disturbed the larvae wiggle violently and drop on silken threads to avoid predation. When larvae are fully mature they generally pupate in the trash at the base of plants or beneath dry leaf sheaths.
The adult moths are difficult to find due to their small size (22mm wingspan), the fact they hide during the day and their dull brown/grey colouration. Adults are most active at dusk when mating and egg laying occurs. Adults do not appear to be attracted to lights, unlike other moth species. The total lifecycle from egg to mature adult takes around 25-32 days.

Damage
The banana scab moth is a severe pest of bananas and can cause up to 100% damage to the bunch if left uncontrolled.
Feeding by young larvae starts as soon as the first bracts lift and usually increases in severity as the larvae grow and move progressively down the bunch as subsequent bracts open. The feeding causes cracking and scarring to the fruit skin, while severe cases can cause disfigurement of fruit as the fingers enlarge. Damage is usually only superficial, where affected fruit is downgraded or deemed unsuitable for the market.
Damage is usually confined to the outer curve of the fingers (the area nearest to the bunch stalk) but, in more severe cases, damage can extend to the stalk, areas between touching fingers, or even extend to cover the whole fruit surface.




Larvae of banana scab moth also consume foliage and can damage plants where a bunch is absent. This leaf damage is worse in varieties such as Lady Finger and Ducasse and is generally not a problem in Cavendish.
For more information contact:
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
13 25 23 or email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been updated as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Banana Weevil Borer mass trapping
Banana weevil borer Mass trapping: A novel design, supporting long-lasting pheromone lures in NSW
Banana Weevil Borer (BWB) is one of the main issues for NSW banana growers. When BWB reach high numbers in the field, they significantly affect productivity by creating a network of tunnels in the corm. This tunnelling weakens the plant and increases the likelihood of blowdowns. BWB infestations affect nutrition uptake, contributing to slow growth, decreased bunch weights and overall poor plant health. NSW growers have successfully designed and implemented mass trapping approaches to deal with this issue, a technique proven to be effective in other countries. Mass trapping reduces pest numbers by luring them, with an attractant, in large numbers to a trap that either kills them or prevents their exit. In this article, we discuss NSW growers’ implementation of mass trapping systems and their successes. Growers who use mass trapping have found it an effective tool for monitoring and successful in reducing BWB pressure and plant damage.


Background
In Australia, typically pseudostem discs or small pitfall traps (less than 500mL volume) are used for monitoring and, to a lesser degree, management of BWB. These strategies are effective in allowing growers to understand their BWB populations and their distribution across their farms. By comparison, international research, and growers, have designed larger pitfall traps (larger than 500mL volumes), to increase rates of BWB capture. These larger pitfall trap designs are possible through the long-lasting pheromone lures which can and historically have also been used domestically in small-scale traps and which can attract both male and female BWB from over 20m away in dry weather. According to an international study (Alpizar et al, 2012), using these large volume mass trapping pitfall traps (with the pheromone lures), at a density of 4 traps per hectare, was 5 to 10 times more effective than traps without pheromone. In this study, corm damage was reduced by half to two thirds after several months of use (from 20-30% corm tunnelling to 10% or less). The resulting reduction of corm damage was shown to increase bunch weights of Dwarf Cavendish (Musa acuminata Colla) by approximately 20%. Trials in Australian growing regions and prominent varieties are yet to be conducted and caution is needed before assuming similar performance outcomes could be attained.
Earlier research by NSW DPI investigated two types of long-lasting pheromone lures (effective for 90 days), to determine the most effective for the NSW region. While both lures are effective at attracting BWB, Cosmolure P160-lure 90 (C.sordidus) is preferred as it does not contain isoamyl acetate, which attracts native turkeys and domestic chickens that damage the traps. Growers who collaborated in the earlier pheromone investigation have continued trapping and over time have developing unique trapping systems using the longer-lasting pheromone.
Mass trapping pitfall design 1 (5L bucket trap)
One of the new innovative methods that growers have developed and implemented is the modified 5L bucket trap. NSW growers have modified a 5-litre bucket to make large volume, mass trapping pitfall traps. To make these traps firstly, several 10-millimetre holes are drilled into the side of the bucket. Next, the bucket is firmly established into the ground. It is important that the drilled holes are flush with ground level and soil ramps need to be made (simply pile soil up into a ramp so that BWBs would be able to walk into the hole and fall into the bucket). Once the trap is established in the hill side, the pheromone bait is set by hanging from the centre of the lid via a piece of wire.




Mass trapping pitfall design 2 (PVC pipe trap)




Another innovative design that NSW growers have adopted is a PVC pipe trap. This trap is made from PVC piping which creates a narrower but deeper trap, more stabilised into the hillside compared to the larger, shallower trap, shown in design 1. In this case, the dimensions are 100mm in diameter, and 500mm in length with the bottom of the trap made watertight with end caps and the top cap left loose to be able to take off. Similar to the pitfall trap there are drilled several holes, 10mm in size, where the trap meets the soil line to allow for BWB to enter. A wire is fixed into the lid or on the side, used to hold the pheromone lure in place. The grower used an auger to install the PVC pipe approximately 300 millimetres in depth into the soil.
Trap maintenance and upkeep
The enlarged pitfall traps require low maintenance and only need to be checked on average once per month or after severe weather events. Emptying of dead BWB will fluctuate as BWB numbers and movements vary throughout the year. According to growers, ensuring the trap stays in place, and maintaining the soil ramps up to the trap are some of the key considerations to keep an eye on and it’s suggested to check these features more regularly.
It has been a suggestion to add soapy water to the bottom of pitfall traps to terminate BWBs once they are in the trap. Furthermore, the soap makes the walls of the trap slippery, preventing them from exiting the trap. However, growers have found that this may not be necessary when the walls of the trap are smooth, as the weevils find it hard to get traction to climb out of the traps.
Cost
The P160-Lure90, Cosomolure (C. sordidus) is approximately $11 per bait (tablet) and lasts 90 days. The current advised density is 4 traps per hectare with one pheromone bait in each trap, totalling 16 pheromone baits per hectare per year. Therefore, currently in 2024, the approximate cost is $175 per hectare, per year. This does not include the material for pitfall traps or labour costs to install and maintain them which needs to be considered. Prices will vary over time. Ensure getting quotes from relevant suppliers before implementation. For some growers, this is a relatively low cost per hectare to substantially reduce BWB numbers throughout a block in NSW. The continued pursuit of trapping innovations reflects the proactive approach NSW growers are taking in BWB management, offering a promising avenue for control. If you are interested in more information about BWB mass trapping contact Steven Norman (NSW DPI Industry Development officer) for assistance.
More information on Banana Weevil Borer:
References
Fu, B., Li, Q., Qiu, H., Tang, L., Zhang, X., & Liu, K. (2019). Evaluation of different trapping systems for the banana weevils Cosmopolites sordidus and Odoiporus longicollis. International Journal of Tropical Insect Science, 39, 35-43
Alpizar, D., Fallas, M., Oehlschlager, A. C., & Gonzalez, L. M. (2012). Management of Cosmopolites sordidus and Metamasius hemipterus in banana by pheromone-based mass trapping. Journal of chemical ecology, 38, 245-252.
This information has been produced as part of the National Banana Development and Extension Program (BA19004). This project has been funded by Hort Innovation, using the banana research and development levy with co-investment from the Queensland Department of Agriculture and Fisheries, New South Wales Department of Primary Industries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.


Chemical treatment of mites
Chemical treatment of spider mites
Restricting the use of chemicals that cause mite population flares
Some chemicals are associated with mite flares. This can be due to several reasons but primarily it is because these chemicals either encourage the mites to lay more eggs (the neonicotinoids, e.g. imidacloprid) or eliminate natural predators (the synthetic pyrethroids, e.g. bifenthrin). Where possible, avoid using these chemicals or if they must be used, time their use to the low-risk periods for mite flares, such as winter.
Correct application of miticides
Firstly, it’s important to check to ensure that live mites are still present and it’s not residual damage that’s still visible. With only a limited number of miticides available to the banana industry, it is important for treatment efficacy and long-term availability of these products that they are applied correctly.
- Avoid using neonicotinoids for control of Banana weevil borer and Banana rust thrips (e.g. imidacloprid), particularly if hot dry conditions are expected. These chemicals can cause mite flares as they encourage mites to lay more eggs.
- Avoid using broad spectrum pyrethroids (e.g. bifenthrin) as these products will remove the predator (beneficial) population. There is also the risk of spider mite populations developing resistance to these chemicals.
- Miticides will not provide instant results and monitoring after spray applications is required as it may take 2-3 days before the mites begin to die.
- Apply miticides in the cooler parts of the day as the leaves will close up during the middle of the day making full leaf coverage difficult to achieve. Mites are generally found on the undersides of the leaves therefore it is important that the leaves are open at the time of application.
- Apply miticides with at least 400 L/ha and up to 600 L/ha of water to ensure good coverage. Poor coverage will result in limited mite mortality and may create chemical resistance problems.
- Rotate between the available modes of action, chemical groups and abide by the restricted number of annual uses for each product to minimise the chance of chemical resistance issues.
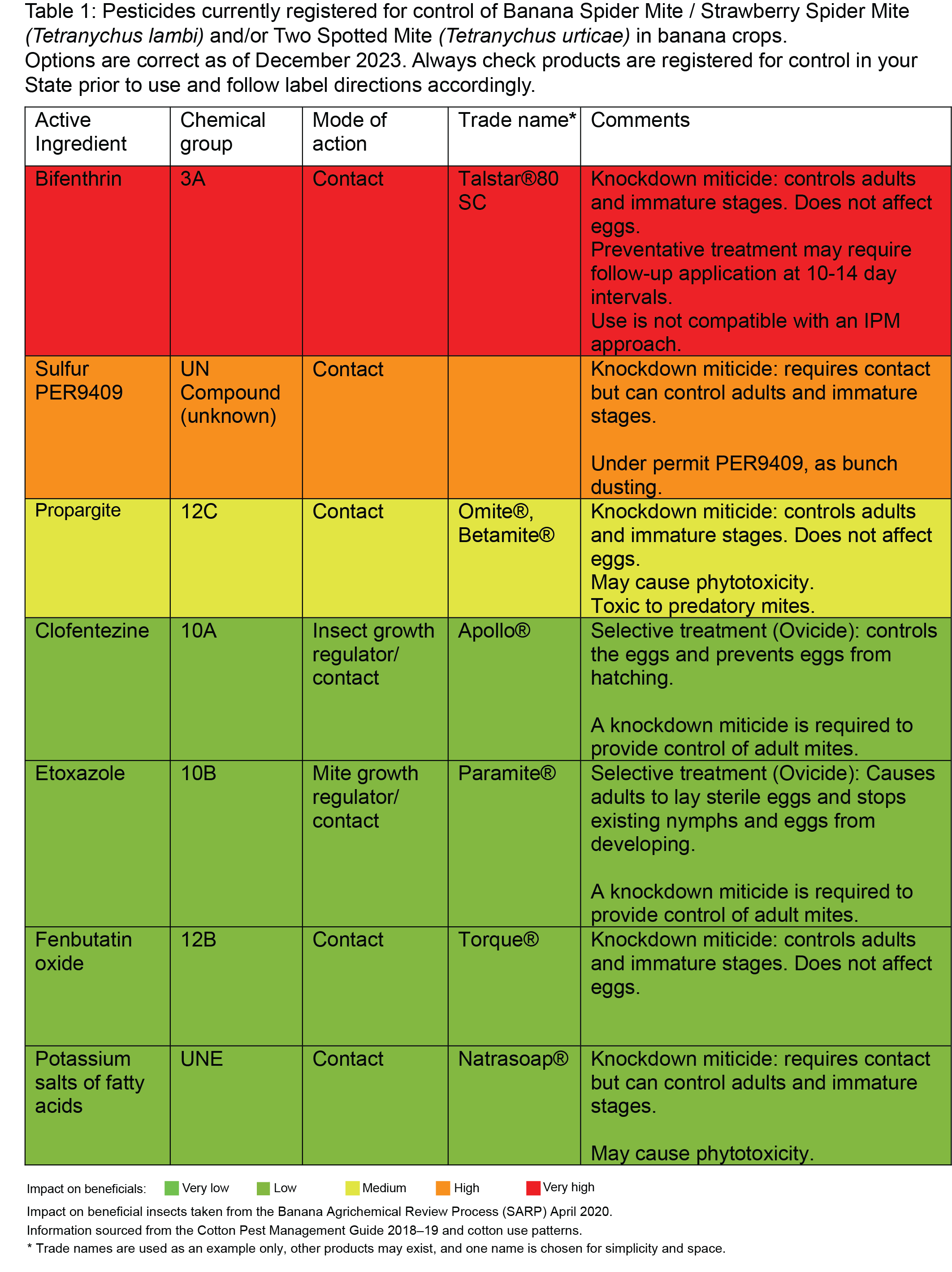
- Knockdown miticides (e.g. fenbutatin oxide) will only control nymphs and adults and therefore may require a follow up application 10–14 days later to control mites that have hatched from the eggs.
- Some miticides are referred to as ovicides (e.g. clofentezine) meaning they only control the eggs, preventing them from hatching. These must be applied with a knockdown miticide to control the adult population. Follow label instructions for resistance management as ovicidal miticides have a history of resistance development if over used.
Actives registered for control of spider mites in bananas
Always check the current registration status of chemicals before use by visiting the Australian Pesticides and Veterinary Medicines Authority website (Click here) and always follow label directions.
For more information contact
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Natural predators of spider mites
Natural predators of spider mites
Natural predators are beneficial insects, that actively hunt and consume specific pest species. Spider mites have many natural predators including lady beetles, predatory mites, rove beetles, and predatory thrips. These natural predators need to be protected through conscientious spray programs (avoiding disruptive sprays) and some can be purchased from suppliers for augmentative releases to address spider mite population flares. Here we investigate two of the key natural predators and how they work to control spider mites.
Stethorus
The small, shiny, black mite-eating ladybird beetle or Stethorus is one of the most important predators of spider mites in bananas. Three species of Stethorus occur in bananas, but the main species is Stethorus fenestralis. All three species appear identical to the naked eye and all species are specialist spider mite predators.
Stethorus numbers increase following mite flares, as mites provide ample food supplies that allow Stethorus’ populations to flourish and eventually bring the mite levels back under control. Stethorus are high-density predators, meaning they are attracted to mite hotspots. Interestingly, both adult and larval Stethorus beetles primarily feed on mites, making them very effective predators against these pests.

The life cycle of Stethorus
There are four distinct stages in the life cycle of Stethorus and it is important to recognise each of these stages. The elongated, translucent to pale brown eggs are laid singly under the leaves, either on or close to the mite colonies. The eggs are about 0.2 mm long and can easily be distinguished from the smaller spherical (and usually more numerous) mite eggs. Mite eggs are not visible to the naked eye, and a hand lens would be necessary to view them in the field.
Larvae are hairy and vary in colour depending on their age. Larvae go through four stages of maturation, each separated by a moult. Young larvae are pale cream becoming dark grey at maturity. Fourth-stage larvae eventually stop feeding when they are about 2 mm long and attach themselves to the leaf where they pupate.
The pupae are black, hairy and about 1 mm long. Pupae may be found anywhere on the underside of the leaf; however, they tend mostly to be found close to or on the midrib. The pupal stage is easily seen on a leaf, as a skin remains after the adult emerges. To determine whether a pupa is alive or is simply an empty pupal skin, smear it gently with a finger. A wet streak will indicate it was alive and if no wet streak is produced, then it was an empty skin.
Adults are shiny black, almost circular beetles about 1 mm long. They also occur on the underside of the leaf. Where there is a high incidence of mite infestation, there may be more than fifty adults under one leaf, although there are usually less than ten when mite populations are in check.
Looking after your Stethorus population
Broad spectrum insecticides are a major cause of mite flares because they destroy beneficial predators like Stethorus. Avoid using these chemicals (e.g. products containing bifenthrin) to control mites. Check your leaves to see if you have Stethorus present and get a gauge on the population levels. Although research specifically in bananas hasn’t yet been undertaken to determine how many Stethorus need to be present to control spider mites, they can keep spider mite populations in check when spider mite pressure is low.



Californicus
Some predatory mites are commercially available for purchase to apply in the field. The more common predatory mite species Neoseiulus californicus (‘Californicus’) is described as an ‘aggressive and robust mite’.
Californicus mites are less than 1mm long and are pear-shaped. Their colouring is dependent on diet but can be clear to pink or orange. Eggs are clear to white, oval-shaped and similar to that of the eggs of the two-spotted spider mite, but distinctively larger. Females can lay up to 4 eggs per day, eggs tend to be laid on the underside of the leaves along veins or on leaf hairs.
Adult Californicus can consume up to 5 adult spider mites daily and can live for up to 20 days. These beneficial insects can even flourish even when prey is scarce, as they are also able to consume alternate food sources such as pollen or other small insects. However, research has shown that reproduction and developmental rates are increased when Californicus exclusively feed on spider mites.
Californicus is known for its resilience to differing environmental conditions. They remain active in both warm and cool temperatures and can survive well in both high and low humidity better than most other predatory mites. However, their optimal conditions are between 16-32˚ C with a relative humidity range of 40-80%. In optimal conditions (30˚ C), their lifecycles can be as fast as 4 days, almost twice as fast as that of their prey. Californicus are also less sensitive to pesticide residues which enables faster re-establishment after chemical applications.
The use of predatory mites as a biological control for spider mites has been trialled on commercial banana farms in Far North Queensland. Some growers release the predatory mites monthly as a preventative treatment for mite flares. For more details on how to release and release rates, contact a commercial biological service provider. The Association of Beneficial Arthropod Producers Inc. has a list of Australian suppliers.
For more information contact:
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Spider mites summary
Spider mites
The banana spider mite (Tetranychus lambi) and the two-spotted mite (Tetranychus urticae) which are both commonly referred to as ‘spider mites’ or ‘red spider’, can cause significant damage to banana leaves and even fruit when present at high levels. They are a common pest of bananas, especially over the warmer spring and summer months. Mites feed mainly on the underside of plant leaves, consuming the contents of plant cells. This permanently damages the leaf and reduces its functionality. With moderate to severe mite damage, fruit development can be delayed and occasionally fruit can be marked with a reddish discolouration towards the cushion end.
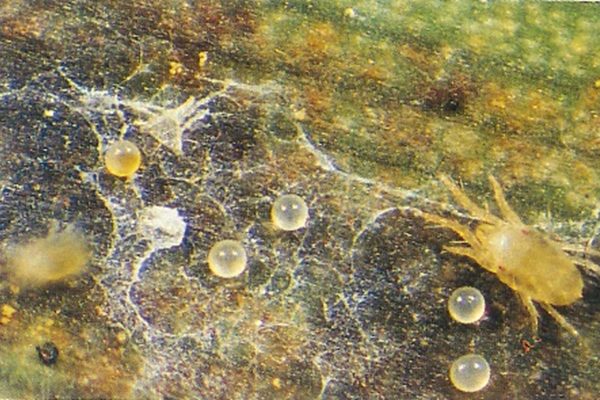
Early detection and the adoption of practices to help minimise spider mite populations will greatly assist in managing this pest. Click below for more information on management options.
More information
This information has been updated as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Spider mites – life cycle and behaviour
Spider mites
Life cycle and behaviour
Both the banana spider mite (Tetranychus lambi) and the two-spotted mite (Tetranychus urticae) are often simply referred to as ‘spider mites’. Both are common pests of a broad range of crops and are widely distributed.
The life cycle and appearance of the banana spider mite and the two-spotted mite are similar. Both mites are typically found on the underside of leaves, only being present on the top side in very high infestations. The main distinguishing feature between the two types of mites is that high populations of the two-spotted mite are always associated with webbing (similar to spiders), while this is absent in infestations of the banana spider mite. Webbing occurs near mite colonies, typically on the underside of the midrib or in severe infestations, down the leaf veins. The two-spotted mite is more commonly found on bananas in South-East Queensland and northern NSW. By comparison, the banana spider mite predominantly is in Far North Queensland and is also identifiable as it is more straw coloured and lacks spots.

The straw-coloured or greenish adult banana spider mites are usually less than 0.5mm in length and are best seen with the aid of a magnified (10X) hand lens. Under good light, the eight-legged adults have a spider-like appearance that can just be made out with the naked eye.
The very small transparent to yellow, spherical eggs are laid singly on the leaf surface and, upon hatching, pass through two nymphal stages before becoming adults. In hot conditions, the life cycle can be as short as seven to ten days.
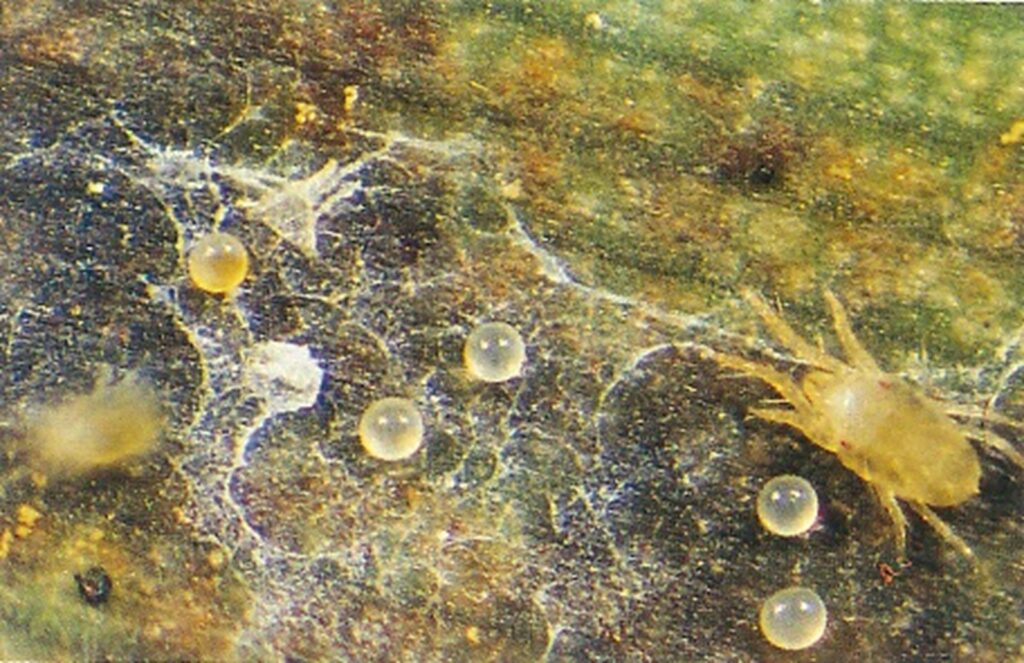
By comparison, the adult female of the two-spotted mite (T. urticae) lives two to four weeks and can lay several hundred eggs during her life.1 Their quick life cycle and the ability of females to produce many eggs, can mean populations build rapidly if conditions are favourable.
Spider mites mainly use wind and small spun lines of web to migrate. The two-spotted mite is known to travel in winds as low as 8 km/h but prefers stronger winds.2 Mites also have the ability to move by walking on or short distances between plants2. Spider mites can migrate at any time, tending to move on when their populations become high, predators become abundant or the quality of food sources declines.
References
- Florida Department of Agriculture and Consumer Services, Division of Plant Industry 2009, University of Florida, viewed 17 January 2022, https://entnemdept.ufl.edu/creatures/orn/twospotted_mite.htm#top
- Seeman, O, Beard, J 2005, National Diagnostic Standards for Tetranychus Spider Mites, Plant Health Australia, Canberra
For more information contact:
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Mite predators and monitoring
Encouraging spider mite predators
Promoting predatory insects to manage mite levels is best done by limiting the use of harmful chemicals, such as broad-spectrum insecticides and miticides, which affect beneficial predatory mites and Stethorus.
Stethorus, the shiny black pinhead-sized lady beetles, are naturally occurring mite predators. They tend to increase in number when spider mite populations are high, as they utilise spider mites as a food source to survive. However, there may be a delay in their population growth due to the initial lack of spider mites.

Predatory mites such as Neoseiulus californicus (Californicus) and Phytoseilus persimilis (Persimilis) can also be purchased for release in your blocks from biological agent suppliers. It has been found that Persimilis may be the more efficient predator in south-eastern Queensland and northern NSW, as it utilises the webbing of the two-spotted spider mite, to locate its prey. While, in Far North Queensland, it’s advised to use the predatory mite Califonicus due to its suitability to the climatic conditions and its effectiveness against the predominant predatory mite, the banana spider mite.
Click here to read more about predators and other beneficial insects
Monitoring mite populations
Mites have a short life cycle which can be as short as 7-10 days during hot-dry conditions and as long as 4 weeks. Over summer months, weekly monitoring is preferable, however, fortnightly is sufficient during cooler, wet conditions. To monitor for the presence of mites, check plants for overall mite damage. The following categories can be used as a guide for the assessment of damage on the underside of leaves.
1 = Low
A few mite colonies on leaves and minor (more than one or two) localised bronzing on the under surface of leaves

2 = Moderate
Mite colonies are scattered but numerous; bronzing is clearly evident on leaves (patchy but starting to coalesce) but the damage is contained within the interveinal areas.

3 = High
Mite colonies coalescing and bronzing damage over most of the leaves.

Applying miticides may be unnecessary if you have low to moderate levels (categories 1 or 2) of spider mite damage and healthy predator populations. To understand mite populations, a X10 magnifying glass is needed to observe all stages of mites (including eggs, nymphs and adults). A healthy predator population may look like finding predatory eggs and nymphs near the mite colonies. This may include finding small black Stethorus beetles. Stethorus are found mainly on the underside of leaves, with their pupae found close to the mid-rib. The presence or absence of mite predators can help you determine the best management strategy moving forward.

If healthy predator populations are detected then your consultant may advise continued weekly monitoring. You may also consider boosting the number of natural predators by releasing the predatory mite Californicus as a hot-spot treatment if only certain parts of the paddock are having mite flares.

In addition to an overall damage assessment, it is also important to take note of the youngest leaf the mites are present on. In general, mites will move up the plant to the younger leaves, particularly as the population grows. By monitoring the movement of mites, decisions about the implementation of control practices to reduce or prevent the severity of irreversible damage to new leaves can be made. In general, the greater the number of mites and the younger the leaves they attack, the more severe the infestation.
It’s important to monitor regularly as spider mite populations can increase rapidly under favourable weather conditions (hot and dry). Therefore, always consider weather conditions before making management decisions. Rain and wet weather will help to keep spider mite populations down. If population levels are high, one rain event may not be enough to reduce spider mite populations sufficiently.
If you have high levels of damage (category 3) and spider mites are present on newly emerged leaves then a miticide treatment will be required to gain control of the population. Click here to view the chemical information below and talk to your consultant for specifics.
For more information contact
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information is adapted from: Pinese, B., Piper. R 1994, Bananas insect and mite management, Department of Primary Industries, Queensland
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


Managing spider mites
Managing spider mites
Causes of mite flares
Broad spectrum insecticides (pesticides), such as bifenthrin, are one of the major causes of mite flares because they remove the beneficial predators that are providing background pest control. The use of such chemicals is not recommended.
Other factors likely to increase the potential for a mite problem include:

Management options
Avoiding the situations mentioned above will greatly contribute to managing spider mite populations. Click on the links below to read more on other activities that will assist include:
For more information contact
The Better Bananas team
Department of Agriculture and Fisheries
South Johnstone
Email betterbananas@daf.qld.gov.au
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.


- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 17
- Next Page »

