Rust thrips – a persistent and pesky pest!
Rust thrips, a tiny pest that can lead to large economic losses, is consistently popping up as a priority pest for banana growers. This is the feedback the banana extension team has been given by growers at recent farm visits. The team is continuing to gain valuable information from growers on issues affecting fruit yield and quality. Discussions so far have highlighted some commonalities across farms, with Rust thrips being raised as the most common issue amongst growers.
What exactly is it though, that makes Rust thrips such a headache for growers? To help answer this, the extension team asked growers and consultants at the 2020 roadshows to share their experience with managing the pest. Here’s what was learnt.

Most growers who attended the roadshows said that they were reasonably satisfied with the level of control they were getting. However, they weren’t happy with the way in which they must achieve it.
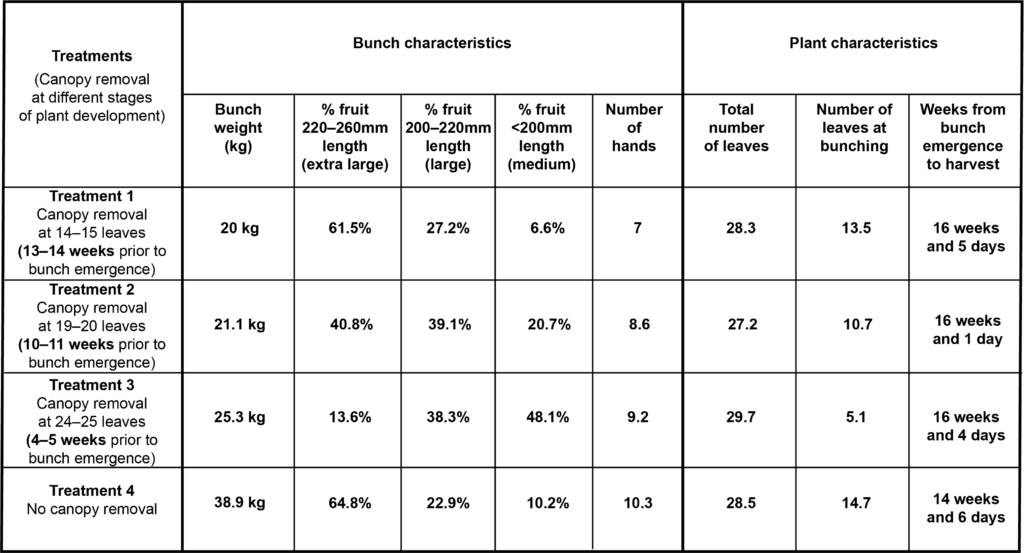
Rust thrips is a persistent pest of bananas, and management practices to control it are on-going and must be undertaken in a timely manner. If not adequately managed, the damage can very quickly become severe and economic losses high. Growers agreed that timely application of chemicals is critical, using the example of bell injection and the need to ensure chemical is injected in the correct position when the bell is still upright. Some growers expressed it wasn’t always easy to be on top of everything that needs to be done, especially given the current labour shortages within industry. Unfortunately, the consequences for growers falling behind in managing this unrelenting pest are almost always high levels of damage to fruit that doesn’t meet market specifications.
Concern was also raised that the current control methods rely too heavily on chemicals, with an over-reliance on their use, with some growers stating they would like to see industry move to alternative softer/biological control options. Workplace health and safety issues surrounding chemical application was also raised, reinforcing the desire to move towards softer biological options. Some growers also indicated their chemical control was no longer effective, suspecting chemical resistance has developed. Rust thrips’ fast life cycle and their relatively sedentary population means they can quickly build resistance to chemicals. This feedback serves as an important reminder for growers to rotate chemical groups as part of their bunch pest management to minimise the risk of chemical resistance.
So where to from here? Project activities included in the project Improved Plant Protection for the Banana Industry (BA16001) include evaluating new chemistries and biological products for bunch pest management. The latest screening of new chemistries, including biological products used for bell injection, commenced in early 2021. Chemical companies will be encouraged to seek registration on any products that perform well in these trials. As part of an Integrated Pest Management (IPM) approach, the project has also investigated cultural controls for Rust thrips. Bunch cover trials have shown that damage levels varied between different coloured bunch covers. Orange covers had significantly higher level of thrips damage compared to all other colours. White covers had the lowest levels of damage. Read more on the bunch cover trials.
While researchers continue to investigate new alternative management strategies for rust thrips, it’s encouraged that growers familiarize themselves with this pest and the current monitoring and control recommendations.
Keep an eye out on the home page for updates on bunch pest research.
Click for more information
This information has been prepared as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.