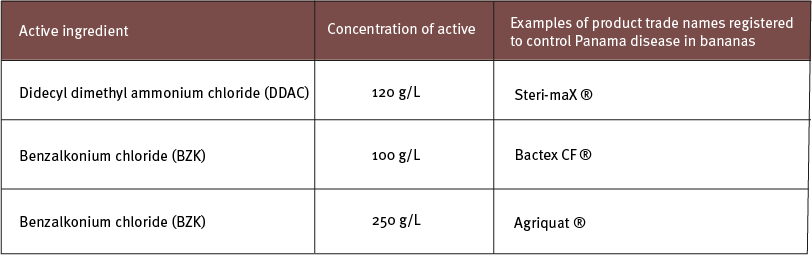
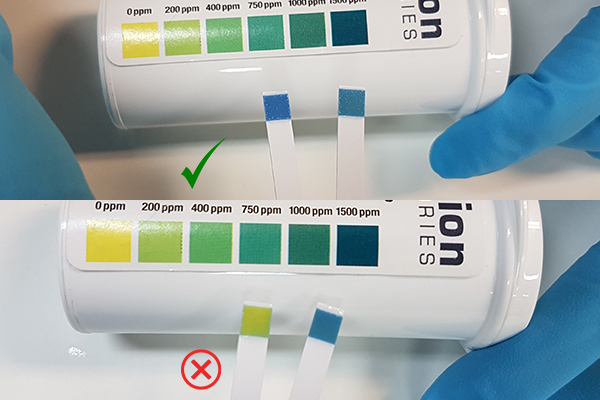
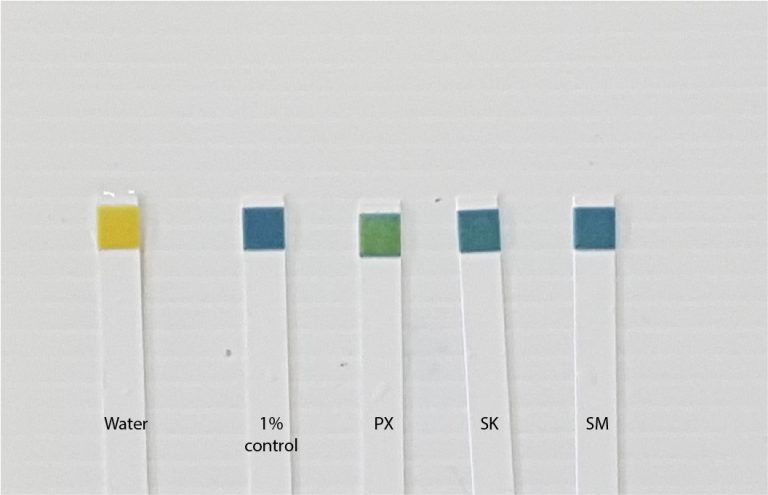
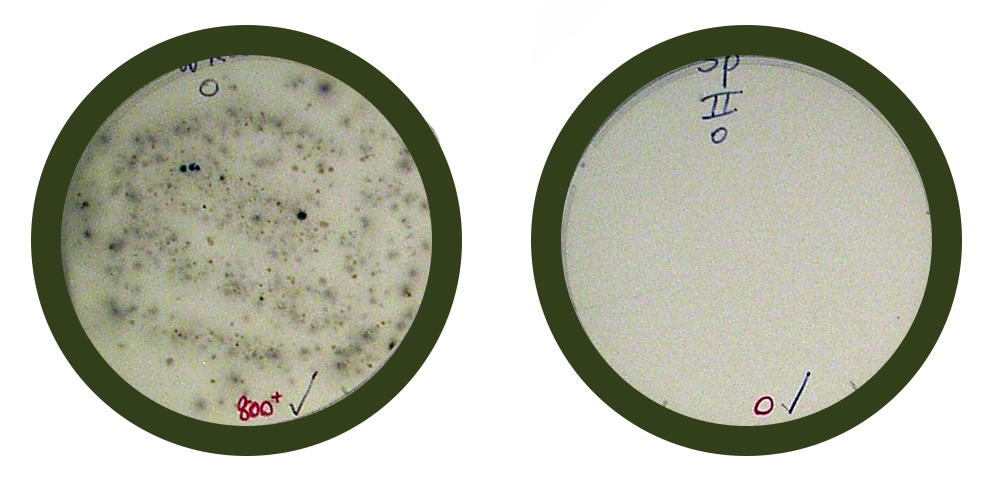
Latest update...
The first ratoon crop is now completed in the variety trial and the results are encouraging with:
- The TBRI Cavendish selection Asia Pacific #3 showing comparable yields and fruit length to Williams over the two crop cycles, combined with Panama disease TR4 resistance much better than Formosana in the NT trials.
- Continued good performance of the four Cavendish selections from Rahan Meristem with yields and finger length equivalent to Williams, with at least two of the selections being significantly shorter in stature.
- The Dwarf Cavendish selection Brier, from the Canary Islands, having yields and fruit length equivalent to Williams, while being significantly shorter in stature.
Click here for more information on first ratoon observations and results
About the trial
Growers are keeping a keen eye on the 32 varieties included in the latest agronomic evaluation at South Johnstone. This is the first step at looking at new introductions that may have commercial potential for the Australian banana industry.
This research forms a significant part of the project Improved Plant Protection for the Banana Industry (BA16001), looking at the agronomic traits as well as pest and disease tolerance of imported varieties. This project provides for 3 variety assessment trials across Australia at Alstonville (previously Duranbah NSW), South Johnstone (Qld) and Coastal Plains (NT), assessing resistance to Panama disease Race 1 and TR4, agronomic performance, cold tolerance and yellow Sigatoka resistance.
Several of the varieties included in the current South Johnstone trial are also being screened in the Northern Territory to determine or confirm resistance to TR4.
Varieties were planted in September 2018 and assessment of agronomic traits will be collected over three crop cycles and a yellow Sigatoka leaf spot screening in the fourth cycle. Several new varieties that have shown resistance to TR4 overseas are included in the evaluation.
With an agreement now in place with the Taiwan Banana Research Institute, it will be possible to progress some of the better performing Taiwanese varieties to on-farm trials. The ability to grow these varieties as part of on-farm trials will allow for a greater number of plants than what is possible at South Johnstone Research Facility.



Varieties being evaluated
- A suite of Taiwanese selections of Cavendish present in Australia. Also included is a selection made in Australia from a former introduction.
- Agro-biotechnology company Rahan Meristem imported four of their elite Cavendish selections into Australia from Israel— Gal, Jaffa and two selections of Adi. The main features include reduced plant stature and large well-structured bunches. These selections are proving popular in various export production zones around the globe. However, these selections are not claimed to have any resistance to Panama disease tropical race 4 (TR4). North Queensland producers that have seen them growing overseas have been keen to see them evaluated by the Department of Agriculture and Fisheries (DAF) for some time. Rahan Meristem own these varieties and have agreed that results from our evaluations can be made publicly available.
- Four hybrids from the breeding program of CIRAD in the French West Indies. Overseas these have shown resistance to leaf disease and Panama disease race 1. Three of these hybrids have shown good resistance to Panama disease TR4 in our Northern Territory trials completed last year.
- Two Cavendish selections from the Canary Islands. These selections of Dwarf Cavendish form the basis of their 400,000 t/yr. export industry to mainland Europe.
A list of varieties being evaluated is now available.
Observations and results
Observations and results are now available for both plant crop and first ratoon.
What's next?
Harvest of second ratoon bunches is now nearly completed. About 10% of the data plants were damaged in early March due to the strong winds brought on by the tropical low (which later developed into Cyclone Niran). Due to the development stage of the Taiwanese Cavendish varieties, these suffered the highest losses. Come November/December the next step will be to nurse sucker the block to synchronize development for leaf spot resistance assessment in the 2022 wet season.
In addition, a new trial was planted at South Johnstone in October 2020. We are evaluating some new varieties which have cleared quarantine since the present trial was established in 2018, along with some improved selections which have been identified in Australia.
Field walks of the trial have provided the opportunity for growers and industry stakeholders to view bunches hanging during the plant and first ratoon crops.

More information...
If you would like further information feel free to contact the Better Bananas team via email at betterbananas@daf.qld.gov.au.
This research has been funded as part of the project Improved Plant Protection for the Banana Industry (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.