Resistance to current post-harvest chemical trials
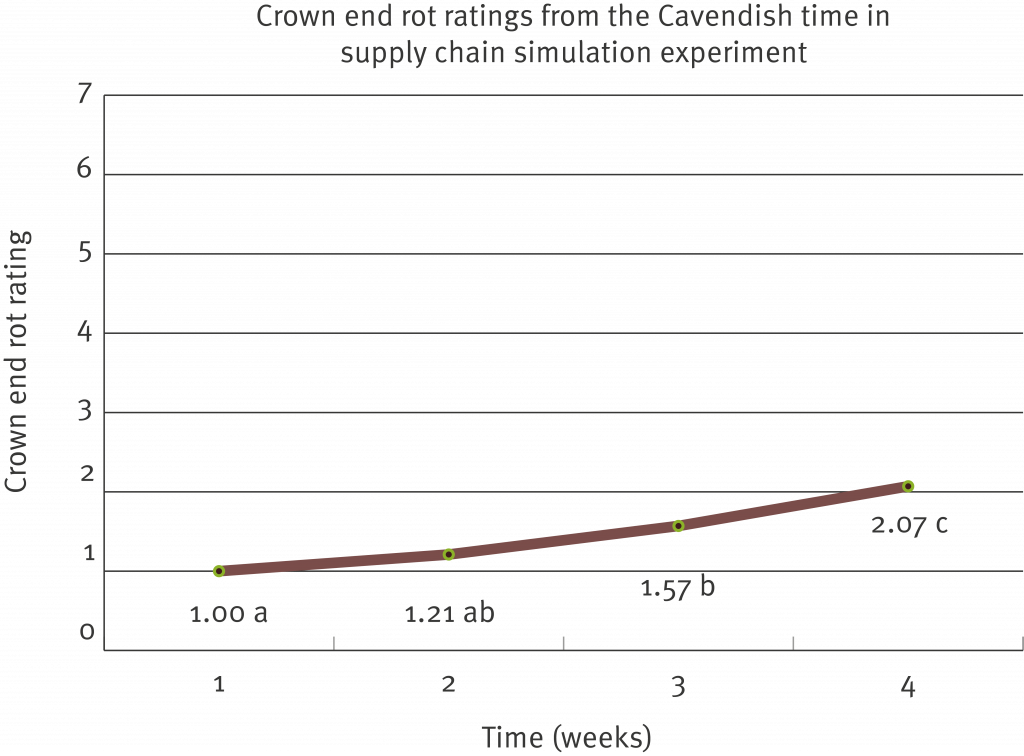
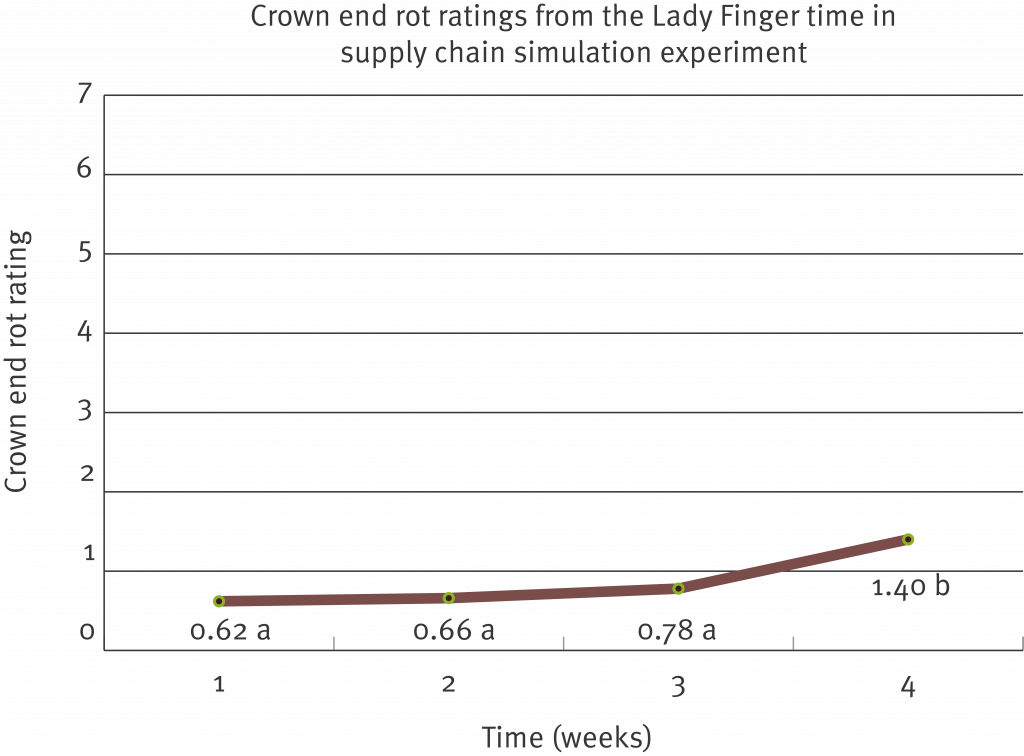
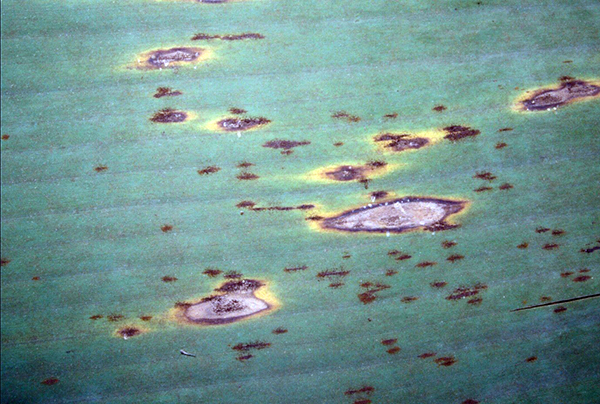
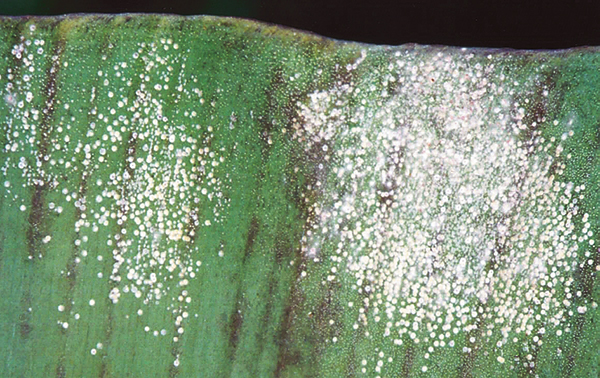
Lab testing used to indicate whether a fungal organism has developed resistance to a particular post-harvest fungicide is commonly termed ‘sensitivity testing’. Sensitivity testing has been carried out on the two registered post-harvest products (prochloraz – Protak® and thiabendazole – Tecto®), against each of the four fungi known to be responsible for causing crown end rot (Fusarium spp., Colletotrichum musae, Musicillium theobromae and Thielaviopsis musarum, commonly known as Chalara).
The fungal organisms listed above were collected from both Cavendish and Lady Finger fruit and from different growing regions including the coastal production area of Far North Queensland, the Atherton Tablelands and northern New South Wales. Where possible the fungi were also sourced from fruit that had not been exposed to commercial management practices (backyard production) in order to provide a suitable comparison.
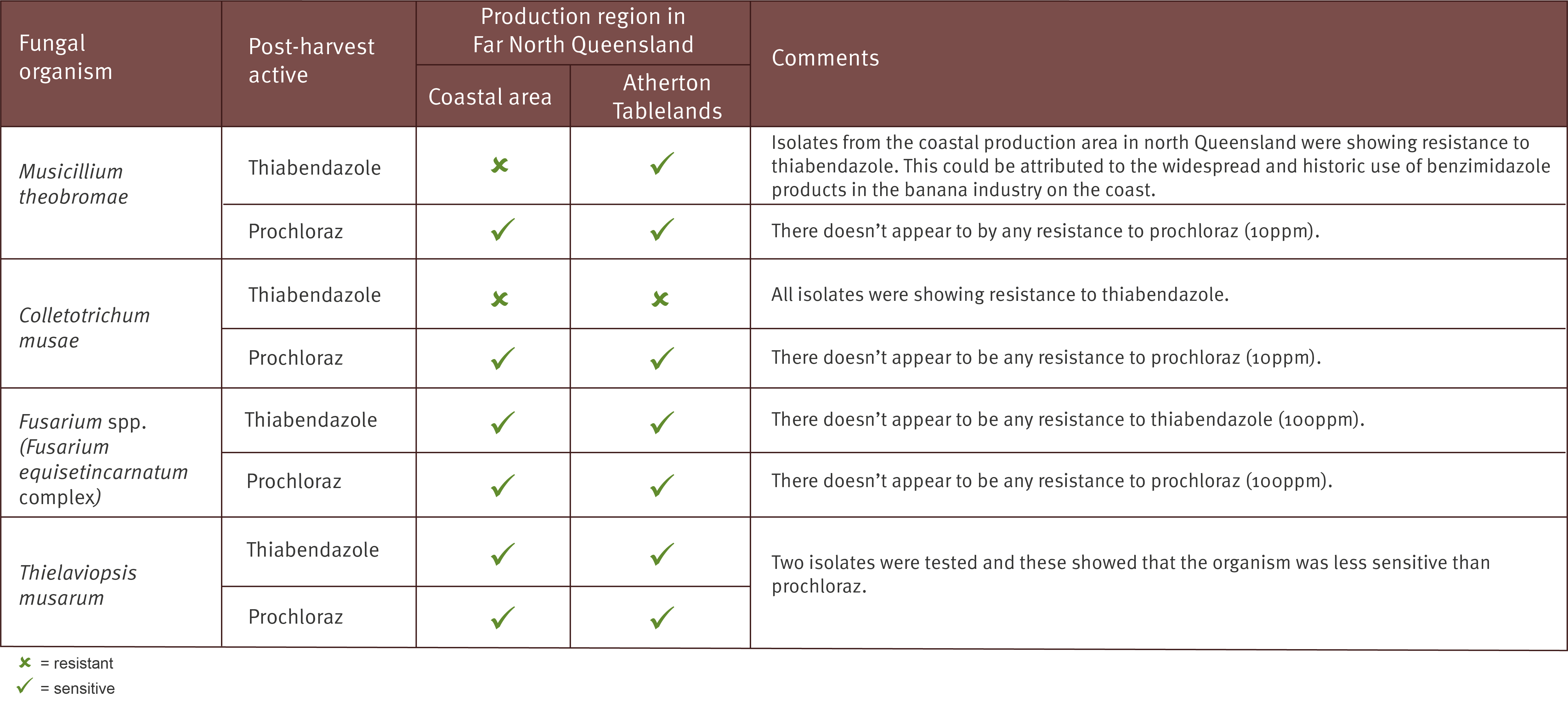
These results show that it may be necessary to change your post-harvest treatment products, depending on the organism/s you are having issues with and the location of your farm.
If you would like more information on this trial contact the better bananas team at betterbananas@daf.qld.gov.au or 13 25 23.
This work is funded as part of the Cause and management of crown rot of banana project (BA13011). This project is funded by Hort Innovation, using the banana research and development levy, co-investment from the Queensland Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.