Field trials - reducing inoculum
The use of high rates of urea to reduce disease inoculum levels of plants infected with Panama disease has been investigated. Researchers have looked at the effect of applying urea to both the soil surface surrounding infected plants as well as infected pseudostems.
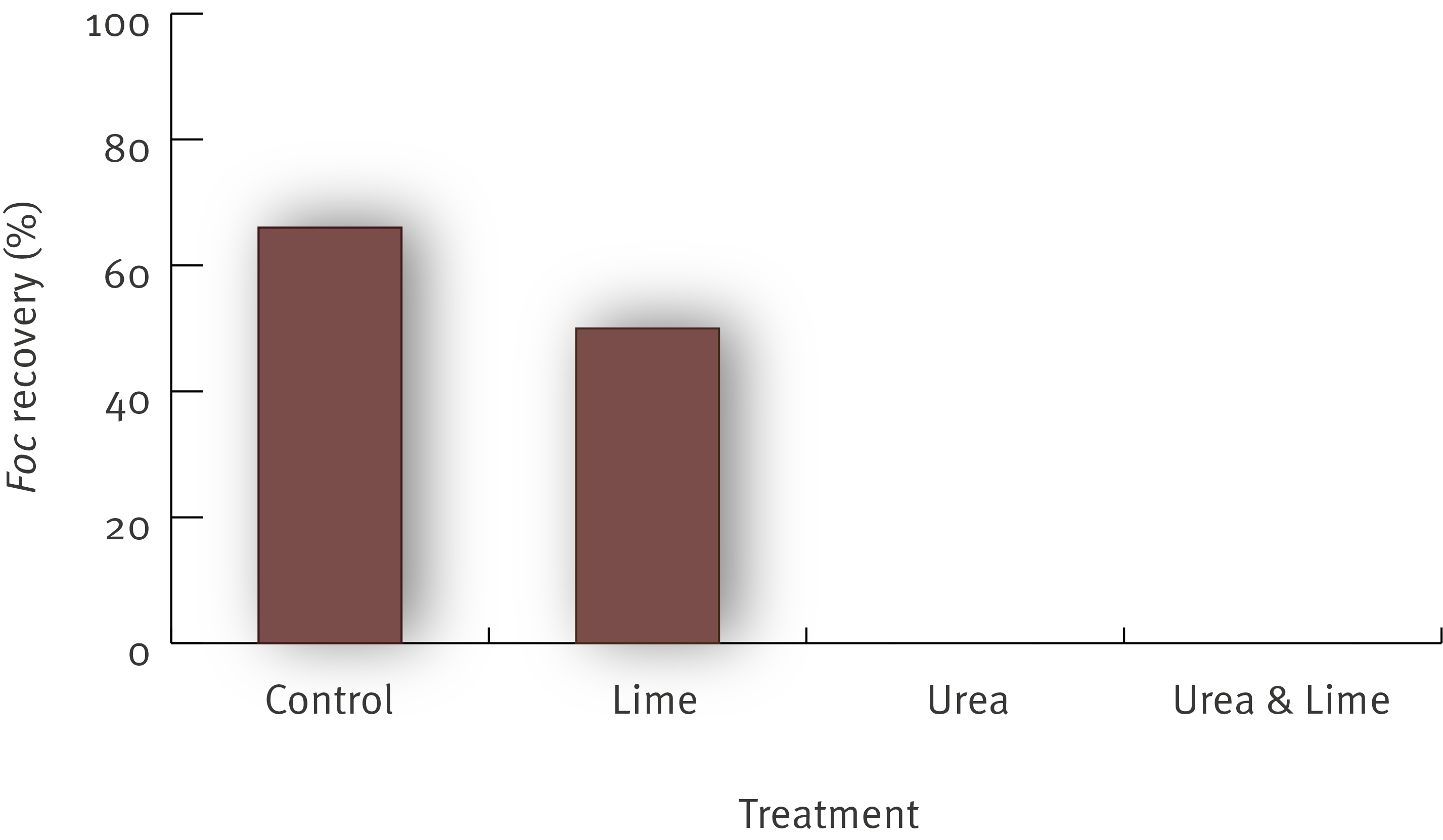
Two field trials conducted at Duranbah in New South Wales used a site infested with Panama disease race 1. In the first field trial, Dwarf Ducasse plants showing symptoms of Panama disease race 1 were cut off at ground level. The growing point was then gouged out and a 1 m2 area was treated around each plant. All gouged corms were treated with 200 g of urea and the 1 m2 areas around the plants were treated with either:
- urea (1 kg/m2) and covered with black plastic
- urea (1 kg/m2), watered in (10 L) and covered with black plastic
- urea (1 kg/m2), Basamid® (500 g/m2), watered in (10 L) and covered with black plastic
- plastic only
- untreated.
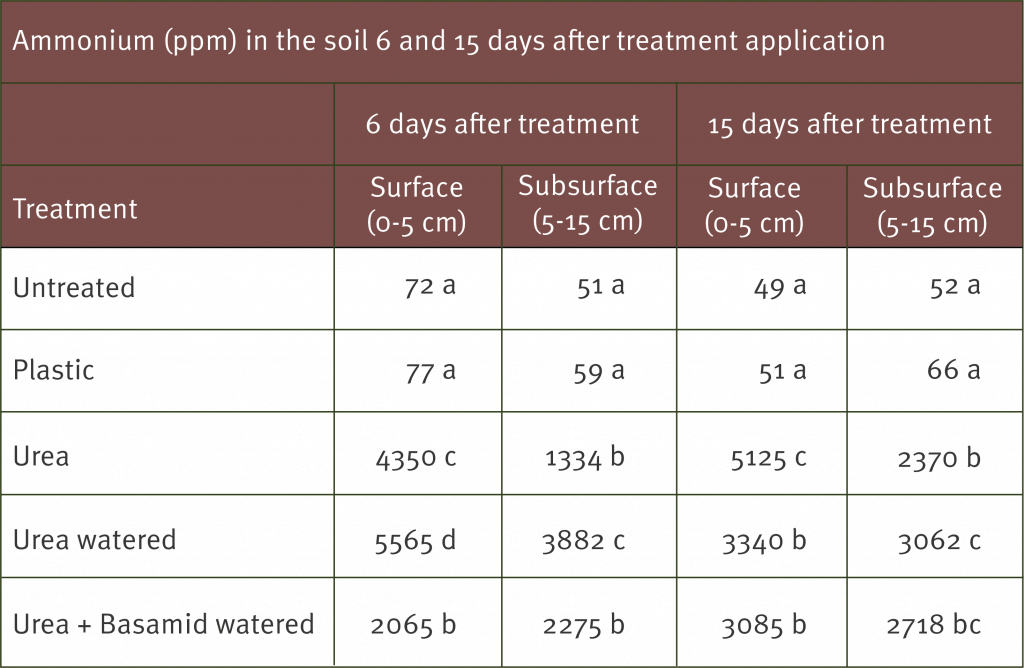
Soil samples were taken from the treatment areas 6 and 15 days after the treatments were applied. The soil was analysed to determine the ammonium content in surface (0-5 cm) and subsurface (5-15 cm) soil and the populations of Fusarium oxysporum in the subsurface samples (5-15 cm).


So what happened under these treatments?
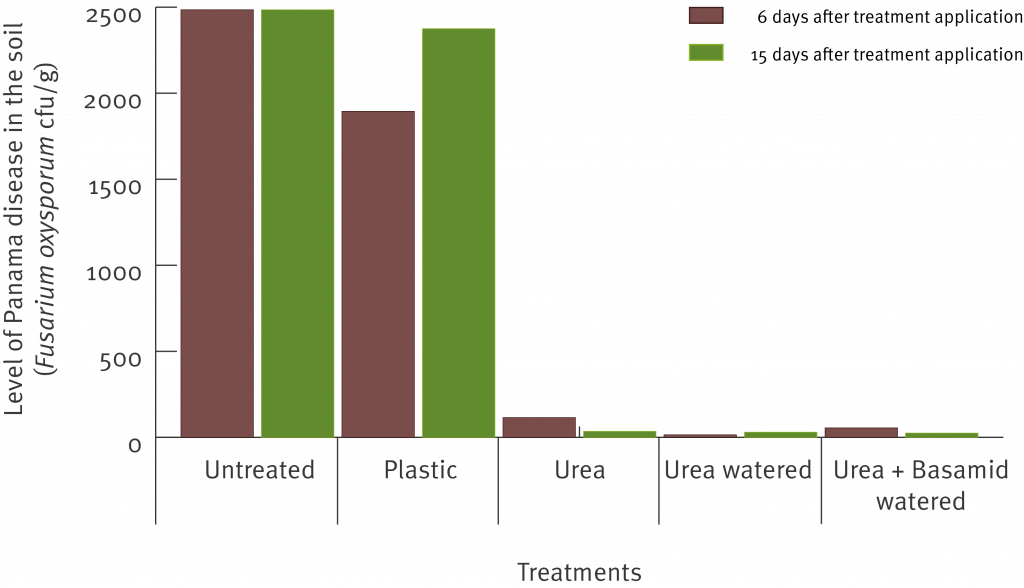
As expected the ammonium levels were significantly greater in the soil treated with urea. As a result the number of Fusarium oxysporum spores in the soil (including the strain that causes Panama disease race 1) measured by the number of colony forming units (cfu) was significantly reduced.
The table below shows the levels of ammonium (ppm) in the soil 6 and 15 days after treatment application.
Means in each column with the same letter are not significantly different at 5% level (P<0.001). For example at 15 days after treatment at surface depth (0-5 cm) there was no significant difference between untreated and plastic. However, there was a significant difference between urea and all the other treatments (15 days at surface depth 0-5 cm).
The graph below shows the significant reduction in cfu under the urea treatments.
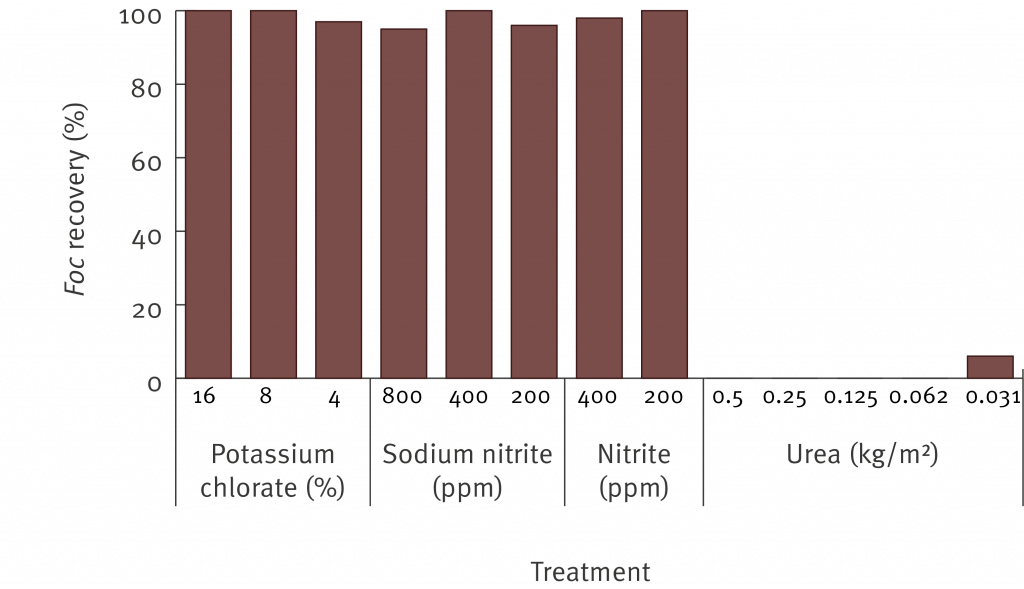
The second field trial investigated the method of cutting up infected pseudostem material and placing it into bags with urea. To test this technique Dwarf Ducasse plants infected with Panama disease race 1 were cut into 15 cm length and placed inside heavy duty plastic garbage bags. Some of the bags included 1 kg of urea and some bags contained no urea. After 6 weeks pieces of vascular tissue were plated out to determine the survival of the fungus. There was no recovery of the fungus that causes race 1 in any of the bags which were treated with urea. There was some reduction in survival of the fungus in the untreated bags, but the pathogen was still recovered.


So what does this all mean...
These field trials confirm that the current method for dealing with infected plants does significantly reduce the amount of inoculum in banana paddocks infested with Panama disease.
For more information about this work contact the better bananas team: betterbananas@daf.qld.gov.au or 13 25 23.
This trial was funded as part of Biosecurity Queensland’s Panama TR4 Program and the Fusarium Wilt Tropical Race 4 – Biosecurity and Sustainable Practices project (BA14013). This project (BA14013) was funded by Hort Innovation, using the banana research and development levy, co-investment from the Queensland Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.