Effects of using different coloured bunch covers on banana rust thrips damage
About the trial
The trial looked into the effect that different coloured bunch covers have on Banana rust thrips damage. The aim is to find non-chemical control methods as part of an Integrated Pest Management (IPM) strategy.
The only chemical treatment applied in the trial was omethoate injected at bell emergence to ensure researchers were only measuring the influence that bunch covers had on rust thrips damage. This meant that there was no damage to the fruit before the bunch covers were put on.
As per commercial practice, bunch covers were placed on bunches when all the bracts had fallen off, approximately a fortnight after bunch emergence. At the time of bagging, the bell and the false hand plus one were removed.
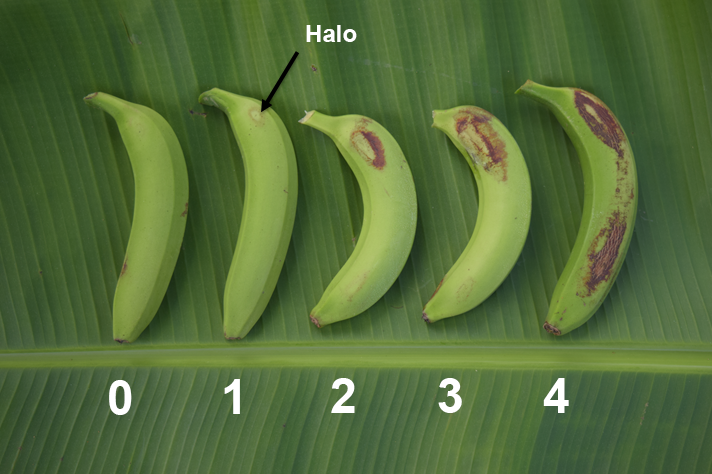
Rust thrips damage was assessed at harvest by examining the surface of 5 central fingers from the top, middle and bottom hands of the bunch.
The presence and extent of damage on the fingers assessed was recorded using a scale of 0 to 4. Rating 1 is defined by a faint halo and is considered the maximum rating for a commercially acceptable level of damage.

Measuring the effect that bunch cover colours had on other fruit quality characteristics was also important to ensure they didn’t have a negative impact. The following fruit quality attributes were therefore assessed at harvest.
- Finger length and diameter
- Colour (Hue, chroma and lightness) of the peel at fruit colour stage 1 and 6
- Bloom (the ‘lustre’ or ‘shine’ of the fruit) was assessed photographically to assess peel reflectance
* Note – Omethoate was registered at the time the trial was conducted and has since been deregistered. Before using any chemicals always check the current registration status and read the product label. Label and permit details can be accessed via the APVMA website, www.apvma.gov.au.
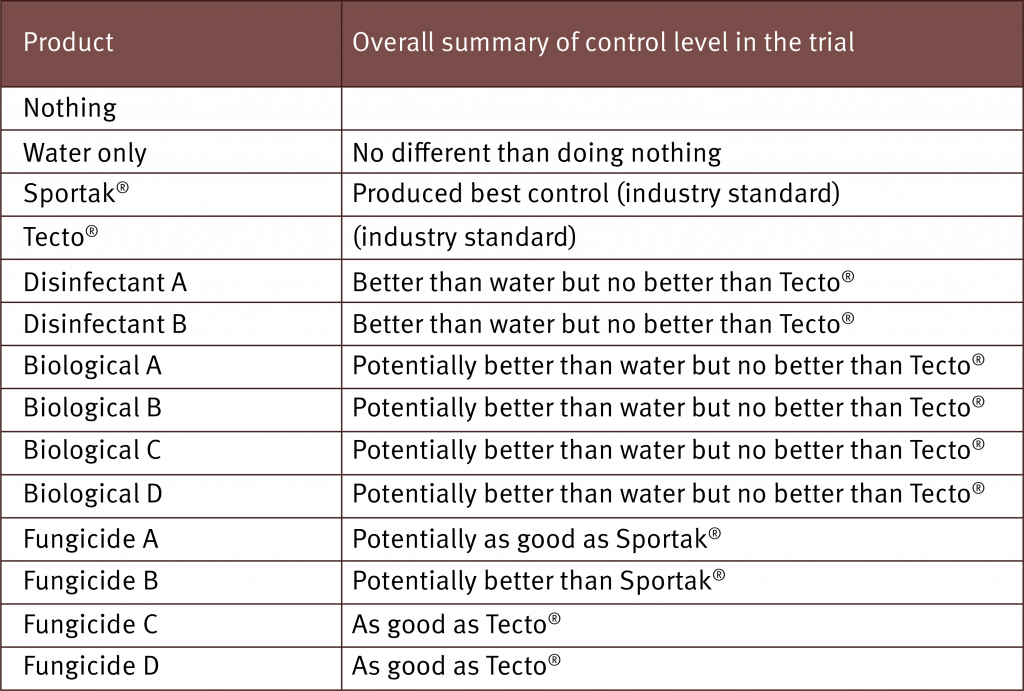
Results from the initial trial indicate that colours do play a role in level of rust thrips damage
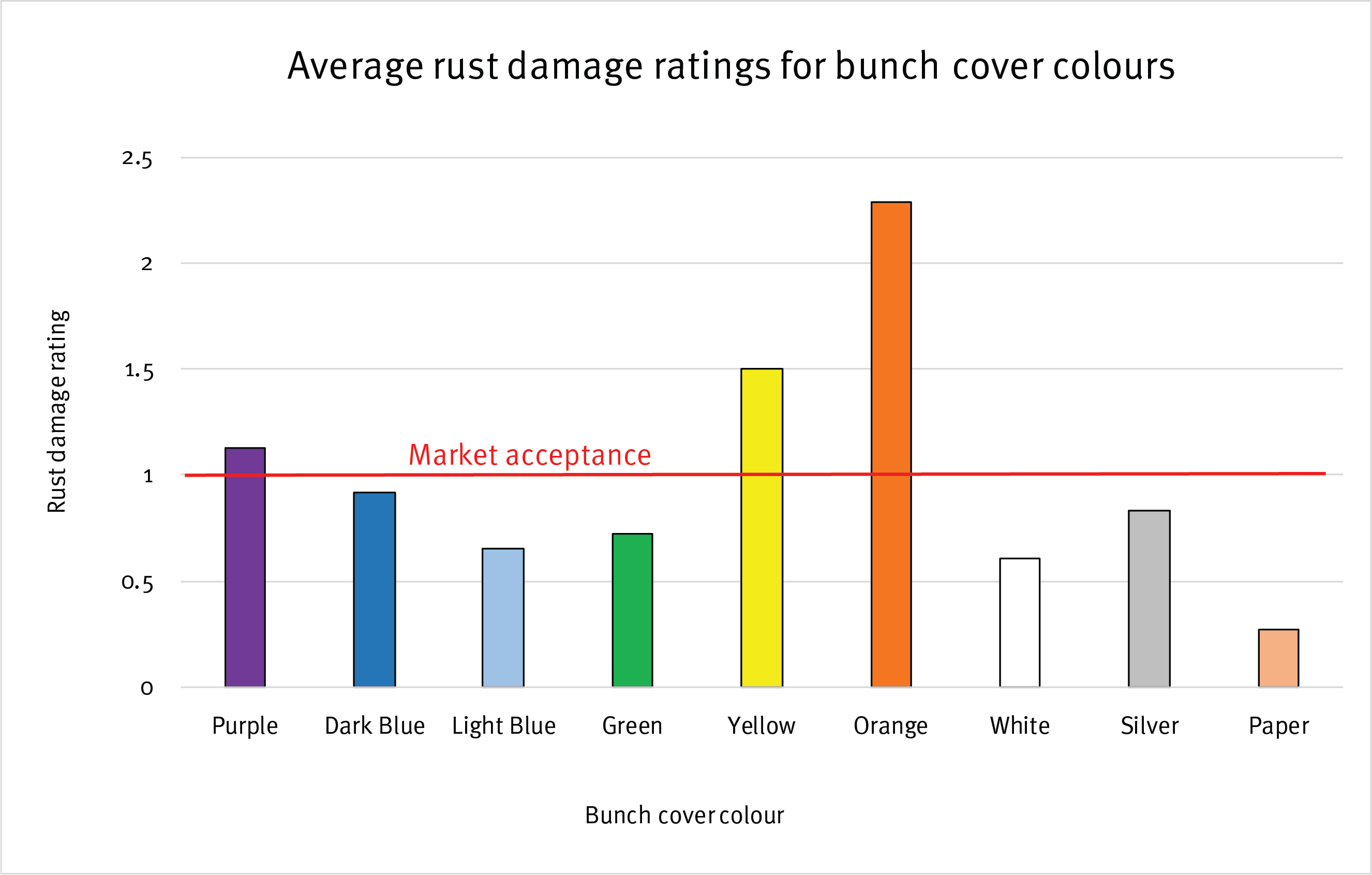
The initial trial results showed a lot of variation in the damage caused by banana rust thrips with respect to different bunch cover colours.
Orange, yellow and purple bunch covers had levels of damage above commercially acceptable levels. Statistically, orange had a significantly higher level of damage compared to all other covers.
The best performer was a paper bunch cover with a polyethylene ‘cloth’ liner. This was the only bunch cover that had a liner in the initial trial. Therefore a new trial is looking at the impact of liners in combination with different bunch cover colours. Light blue and white produced similar low levels of damage as the paper bag.
Results also indicate that bunch cover colours have no statistically significant effect on finger length, diameter, colour and bloom.
Growers should consider using bunch cover colours with low thrips damage rating
Based on the initial trial results, growers should consider using bunch cover colours that scored a low thrips damage rating. Further work is underway to expand these results by testing new colour and liner combinations. Results of the latest trial will be available to growers in the coming months.

If you would like further information on this trial, contact the Better Bananas team.
This research is funded as part of the Improved Plant Protection for the Banana Industry Program (BA16001), which is funded by Hort Innovation, using the banana research and development levy, co-investment from the Department of Agriculture and Fisheries and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.