Common pests, diseases & disorders of banana
Bananas can be impacted by a range of pests, diseases and disorders. Having appropriate management practices in place is essential for ensuring good quality fruit. Below is some general information on some of the major pests, diseases and disorders of banana. It also includes summaries of recent trial work undertaken by researchers from the Department of Agriculture and Fisheries.
If you are having problems identifying what is causing damage to your crop check out the Better Banana’s problem solver section here.

Pests
Banana flower thrips (Thrips hawaiiensis)
Banana rust thrips (Chaetanaphothrips signipennis)
Banana scab moth (Nacoleia octasema)
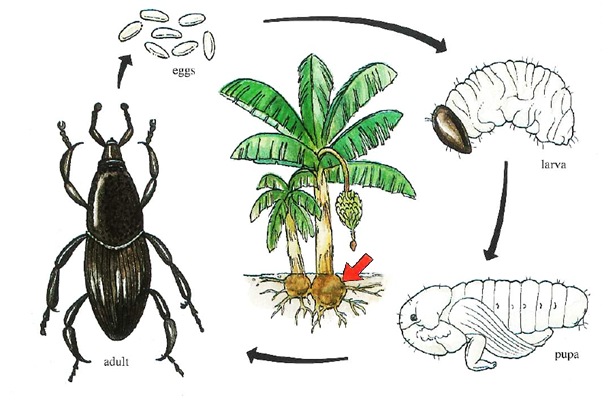
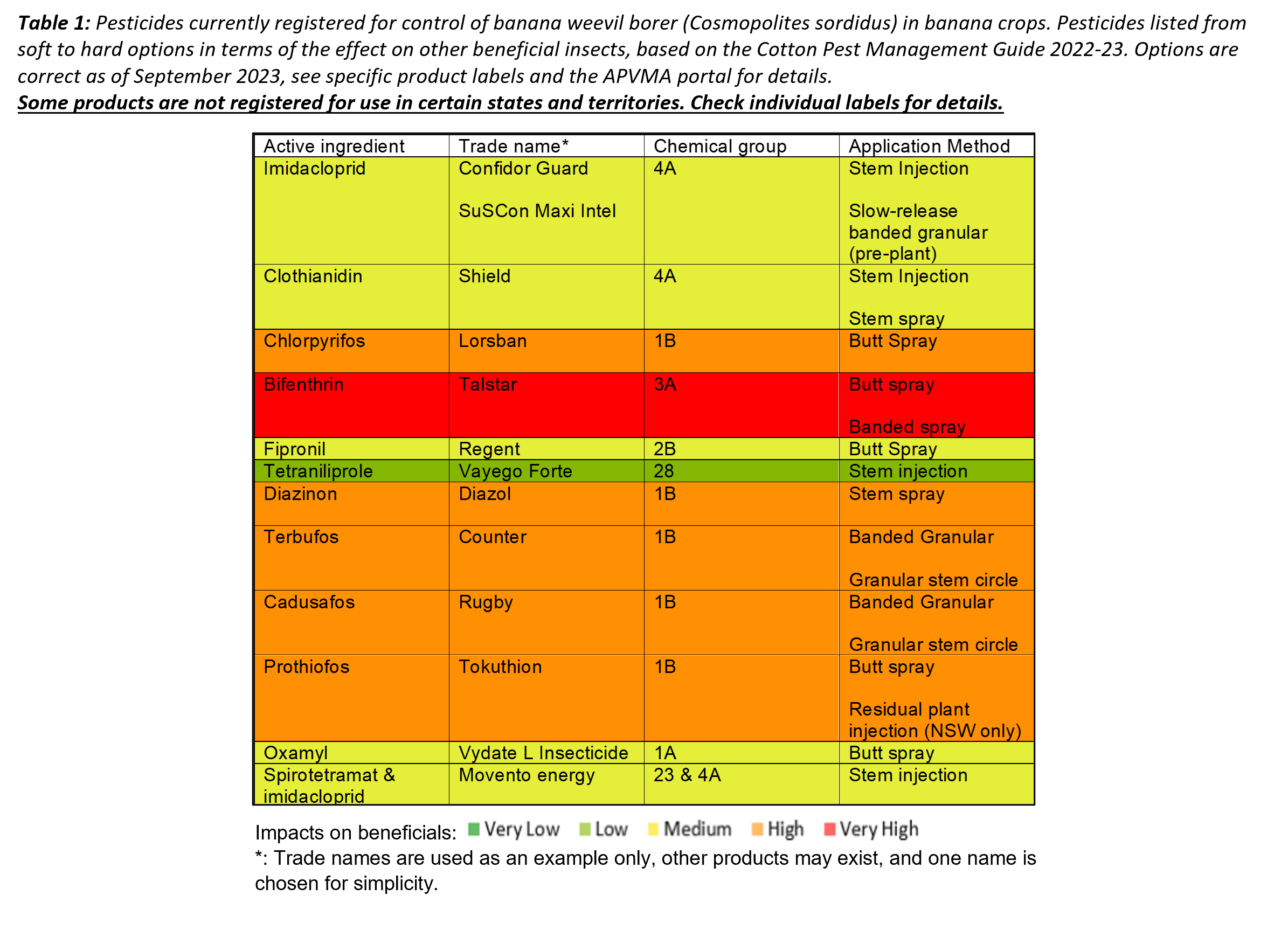
Banana weevil borer (Cosmopolites sordidus)
The banana spider mite (Tetranychus lambi) and the two-spotted mite (Tetranychus urticae)













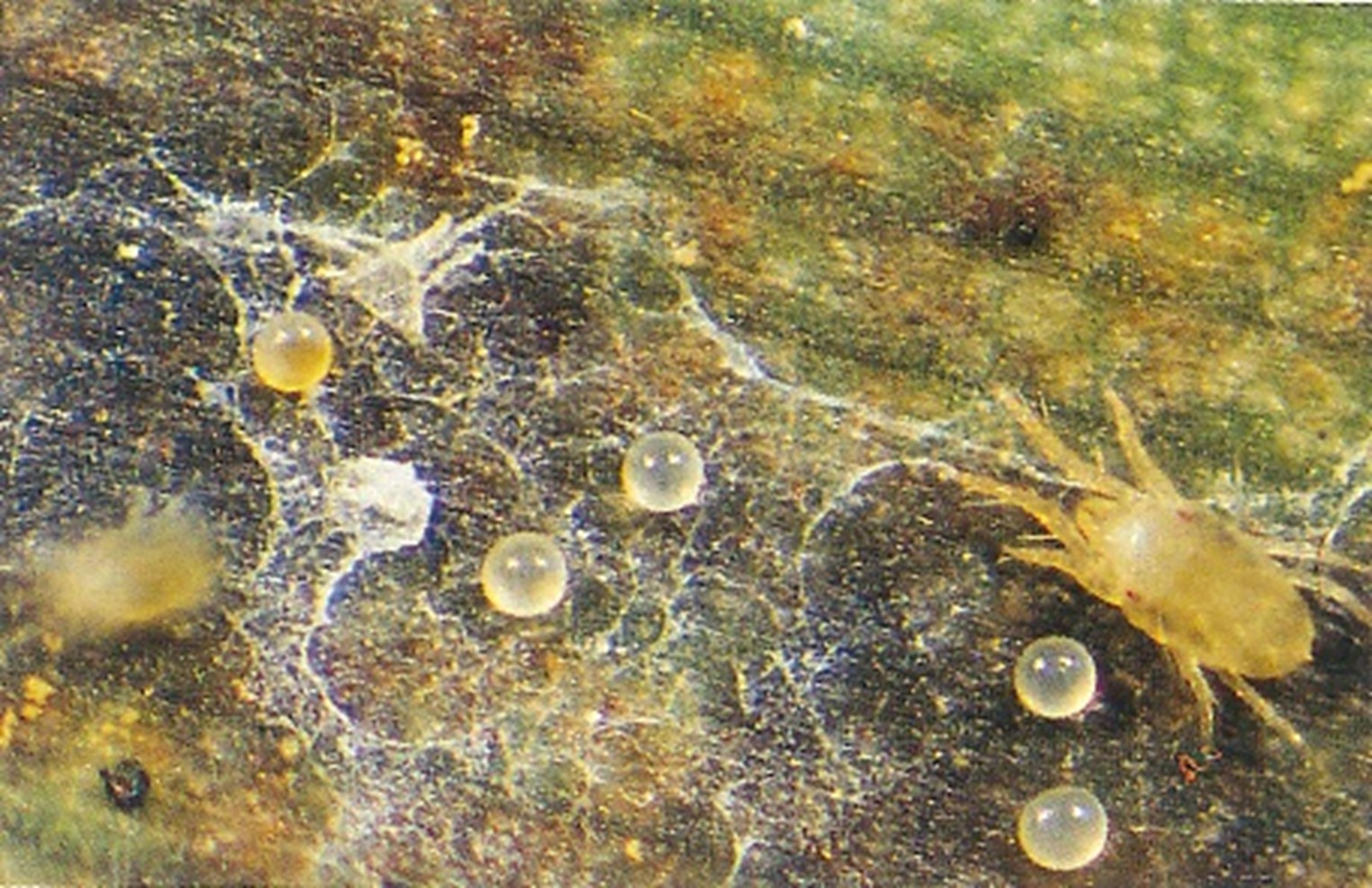
Diseases
Crown end rot (Several species including, Musicillium theobromae, Fusarium equiseti-incarnatum species complex, Colletotrichum musae and Thielaviopsis musarum)
- Crown end rot summary page
- Protecting crowns improves fruit quality for Sellars Bananas – Better Bananas
Crown end rot trial work
Panama disease (Fusarium oxysporum f.sp cubense)
Panama disease trial work








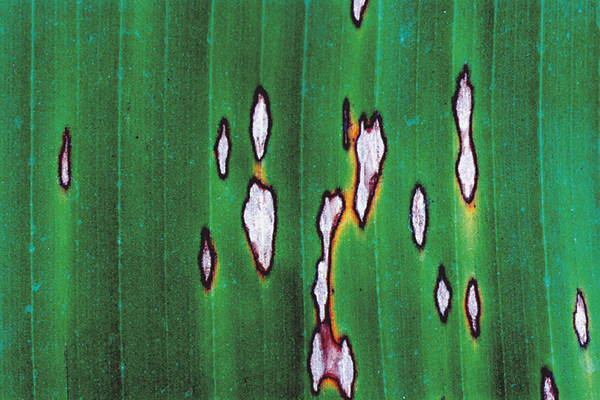
Physiological issues and disorders


For more information contact:
The DPI Banana Extension Team
Department of Primary Industries
South Johnstone
13 25 23 or email betterbananas@daf.qld.gov.au
This information has been developed as part of the National Banana Development and Extension Program (BA19004) which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Agriculture and Fisheries.