New test helps product screening for Chalara management
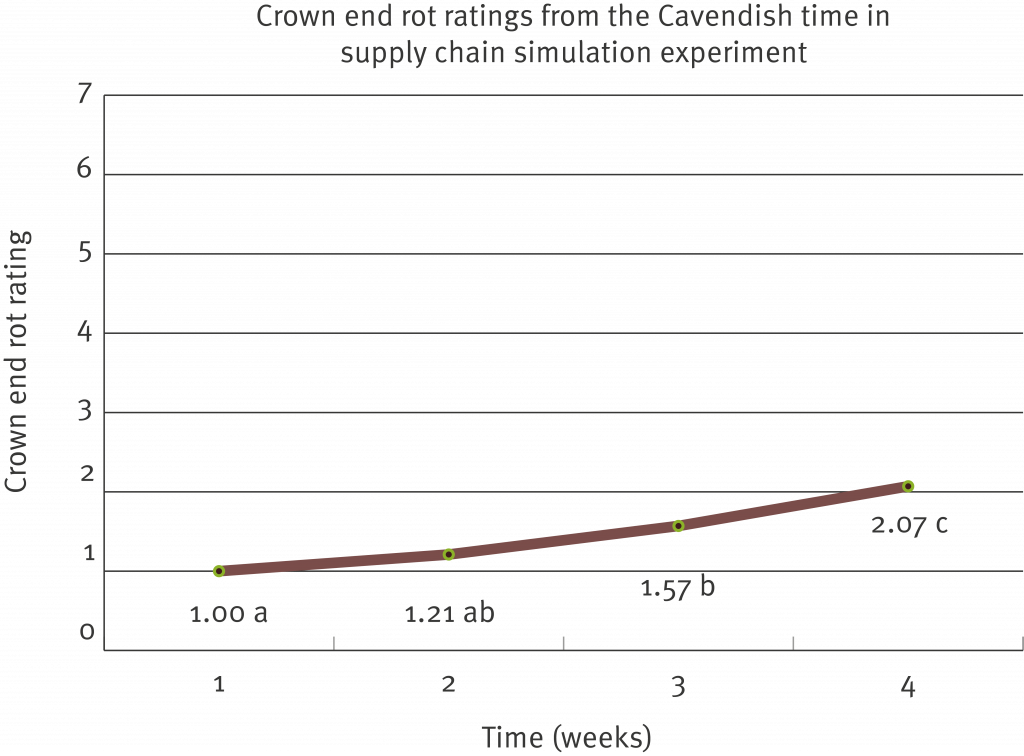
Multiple fungal organisms are known to cause Crown end rot (CER) in bananas. The following research is focused on the more serious form of CER commonly known as Chalara where the rot extends into the fruit (caused by Thielaviopsis musarum). Disease symptoms are typically observed in the supply chain during cooler periods of the year (winter). Chalara is sporadic in occurrence, making it difficult to conduct research trials with the disease. Department of Agriculture and Fisheries researchers have now developed an inoculation technique that mimics the development of Chalara in the supply chain, enabling researchers to screen and evaluate alternative management options.
There are two post-harvest fungicides currently registered for use in Australia to help manage CER. Although these treatments are effective against the fungi that cause CER, growers have expressed a need for non-chemical options for managing the disease, particularly those with organic status.
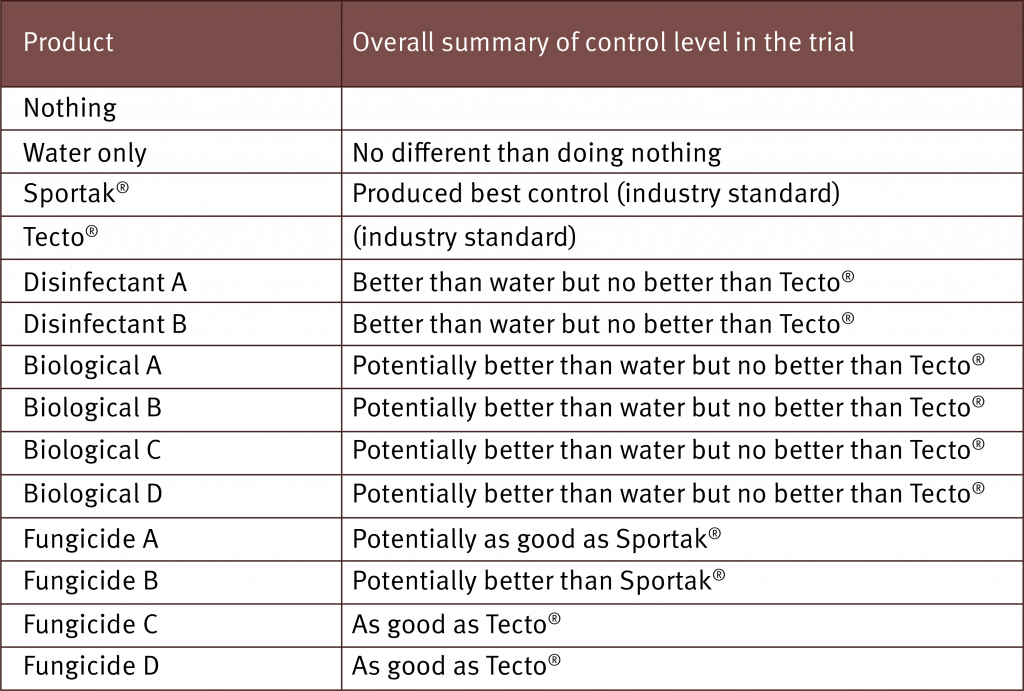
The inoculation technique has now been used to determine efficacy of the currently registered fungicides, alternative fungicides and biological products.
Results
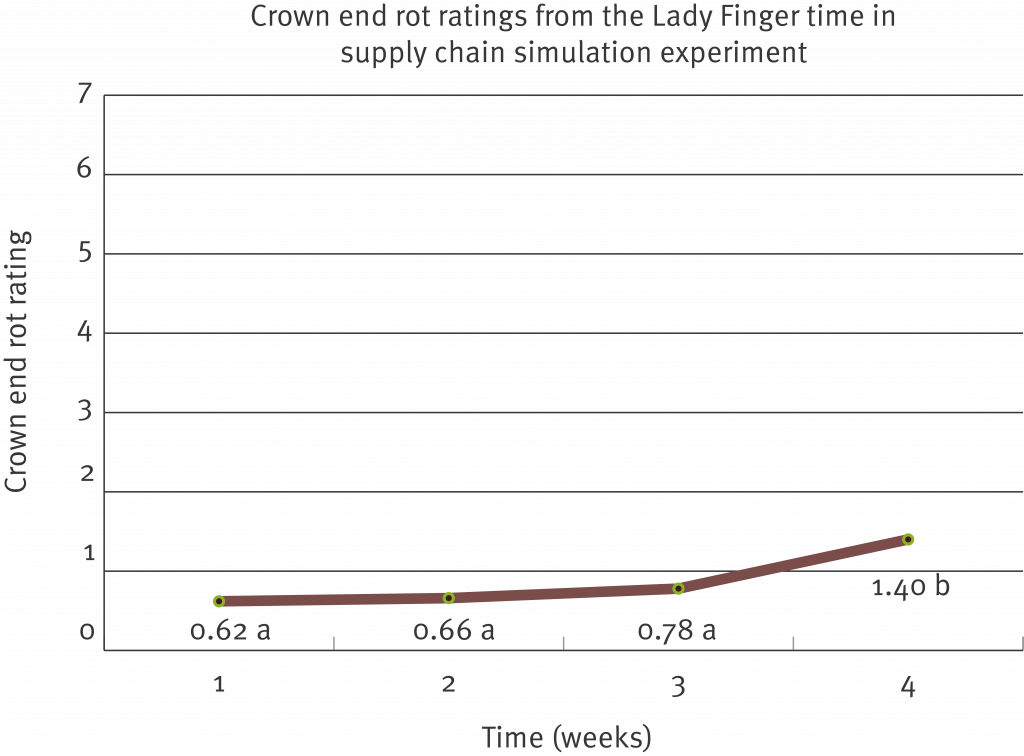
Overall, the inoculation technique developed is rapid and reliable and the results are reproducible. Even though the technique was specific for Chalara (T. musarum), crown mould assessments were also obtained. Ideally a successful test product should have efficacy against T. musarum and the range of fungi that cause crown mould.
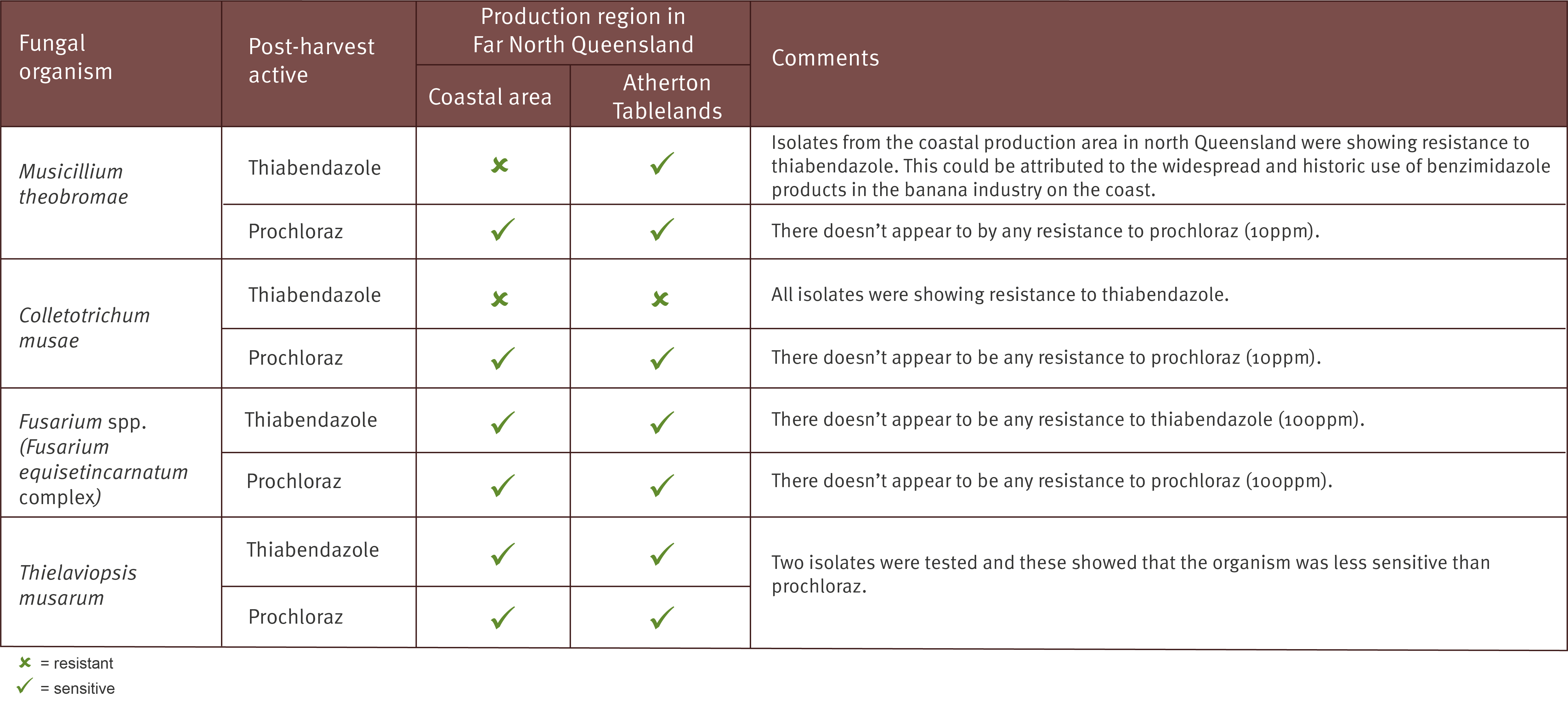
Prior to conducting this research there was only anecdotal evidence that the current registered projects had efficacy against T. musarum, but this has now been confirmed, with both Tecto® and Protak® effective in halting the development of Chalara. Results also showed that some biological products are capable of managing Chalara and reducing levels of crown mould.
Participating companies have been supplied the results for their products. They can use the results to support registration applications and/or determine which products are worth investing in further trials. It is hoped this work will lead to product registration adding alternative management options for growers.



Remember...
Before using any chemicals, always check the current registration status and read the product label. Label and permit details can be accessed via APVMA website: www.apvma.gov.au
This work was undertaken as part of the ‘Enhancing the outcomes of BA13011-Crown end rot investigations’ funded as part of Department of Agriculture and Fisheries’ Horticulture and Forestry Science development funding.