First ratoon results - agronomic evaluation of Lady Finger-like varieties
(Trial established December 2022)
Exploring new options for Australia's Lady Finger growers
By Katie Robertson, Jeff Daniells, Sharan Muthukumar and Carole Wright (December 2025)
Latest update...
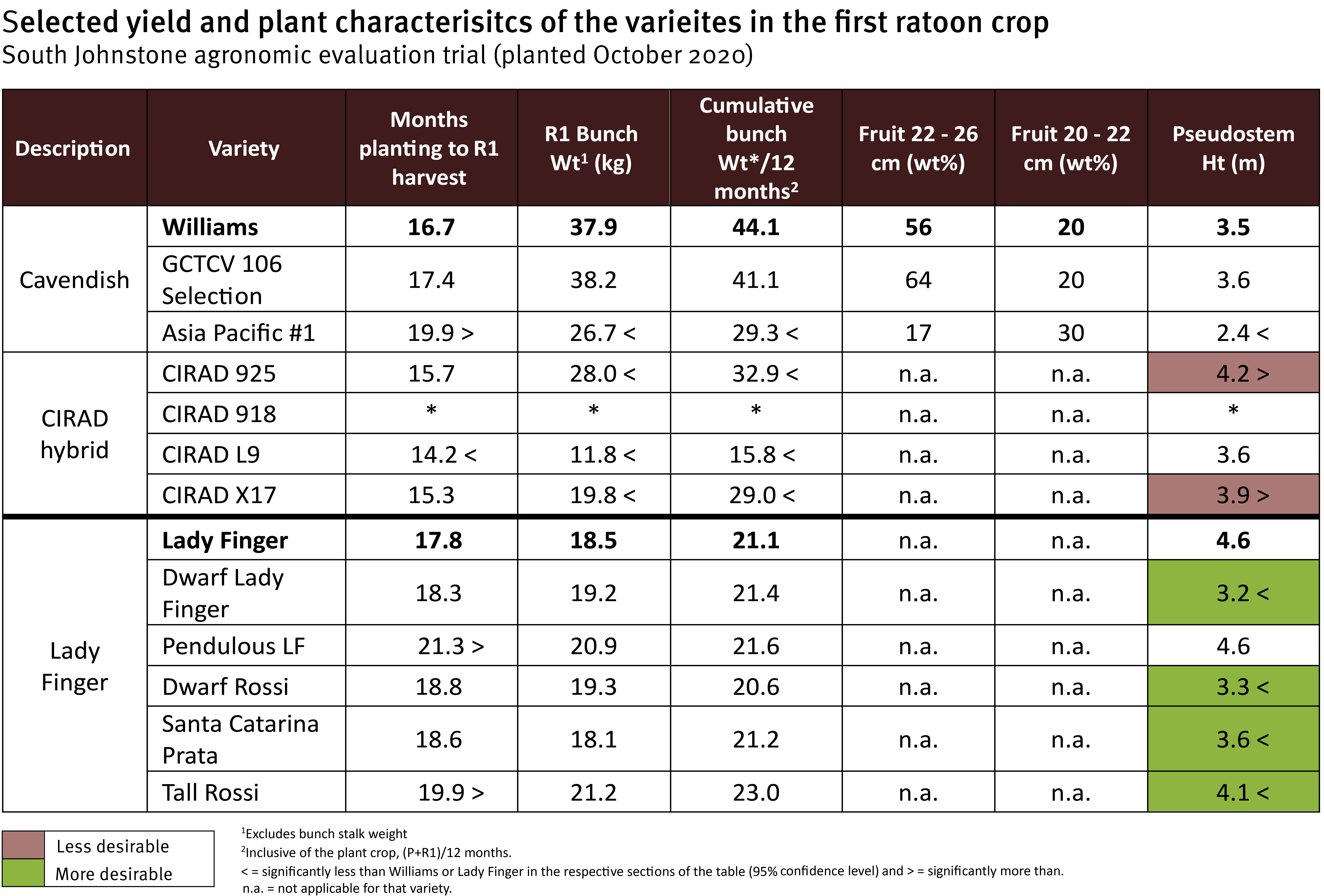
The agronomic performance and taste of several Lady Finger-like varieties were evaluated over a plant crop and first ratoon at the South Johnstone Research Facility. The semi-dwarf Lady Finger selection, SCS451 from Brazil, looks promising with its high yield, short stature, pendulous bunch and desirable eating characteristics. On-farm trials are currently in progress on the Atherton Tablelands, examining the Fusarium wilt Race 1 resistance of several of these varieties.
As Fusarium wilt Race 1 continues to spread across Lady Finger production areas in Queensland and New South Wales, the search is on for alternative, disease-resistant varieties which could help reinforce this small, yet important share of Australian banana production. Under the project BA21002 ‘New varieties for Australian banana growers’, the agronomic performance of several Lady Finger-like varieties (Lady Finger selections, Lady Finger hybrids, Silk hybrids) with purported resistance to Fusarium wilt Race 1, were evaluated over a plant crop and first ratoon at the South Johnstone Research Facility. In-house taste panels were also conducted to gain preliminary feedback on the fruits’ eating quality and see how they align with consumer taste preferences. Staff were asked to rate their overall eating experience of the fruit on a scale of 1 – 9 (dislike extremely – like extremely) and leave any comments to further explain their rating.
The plant crop results are summarised in the August 2024 edition of Australian Bananas (pp. 22 – 23), which highlighted and explained the offtype issues identified in the control Lady Finger plants. This problem subsequently left only one plant for comparison with the new varieties. However, earlier studies on the research station suggest the data from this plant is agronomically similar in terms of height and finger length, but cycle time was more rapid than what has previously been observed (which would be more comparable to SCS451).
Key findings
Some key findings from the completed trial are as follows:
-
Japira, Pacovan Ken, Pacovan and SCS451 produced the heaviest bunches, with the latter three yielding the highest across a 12-month average (cumulative yield) when cycle time was accounted for.
-
SCS451 was shorter in stature than the other varieties at 3.5 m, followed by Platina at 4.0 m. Princesa and Tropical were comparable to Lady Finger at 4.3 m, while the remaining varieties were taller (the tallest was Japira at 5.4 m). As was noted in the plant crop, the increased height made several aspects of crop management more challenging.
-
Japira had the longest fruit, measuring 24.6 cm on the third hand, while Princesa and Tropical had the shortest fruit (16.8 cm).
-
Despite Tropical bunches emerging in winter in the first ratoon, it had the shortest fruit filling period of 107 days. However, it took longer to reach bunch emergence, resulting in it having the slowest cycle time overall.
-
Bunches of the SCS451 threw well clear of the throat which contrasted with the related Santa Catarina Prata that often exhibits various degrees of choke-throat symptoms. Pacovan and Pacovan Ken also exhibited more pendulous bunches than Lady Finger.
-
The taste ratings improved for all varieties in the first ratoon compared to their relatively poor performance in the plant crop, except SCS451 which maintained a consistent score of ‘like moderately’. It was enjoyed for its balanced sweetness/acidity and firmer texture.
-
In the case of Princesa, it jumped from having a rating of 4.1 (dislike slightly) to being on par with SCS451 in the first ratoon with a ‘like moderately’ rating. One explanation for this is that fruit in the plant crop contained several mature seeds which negatively impacted the eating experience (although comments were also made on it having an unpleasant flavour). However, no seeds were present in the first ratoon fruit, and the texture was positively described with words like ‘soft’ and ‘creamy’. Several comments were made about its ‘unique’ and ‘unusual’ flavour with some describing it to have notes of passionfruit or papaya.
-
In the first ratoon SCS451, Princesa and Pacovan achieved comparable taste scores to Lady Finger (‘like moderately’).
-
Pacoua and Pacovan Ken remained the least favoured varieties out of those tasted, primarily attributed to their softer, ‘slimy’ texture.

Several of these varieties are being screened to confirm Race 1 resistance in two on-farm trials on the Atherton Tablelands. Look out for an update on plant crop results from those trials in a future issue of Australian Bananas.
Results
Table: The yield and plant characteristics along with the score of the fruit’s overall eating experience. Mature bunches from the varieties Japira and Tropical were unavailable at the correct time to include in the taste panelling for the first ratoon.



More information...
This research has been funded as part of the project New varieties for Australian banana growers (BA21002), which is funded by Hort Innovation, using the banana industry research and development levies and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture. The Queensland Government has also co-funded the project through the Department of Primary Industries.